Evolution of vertebrate opioid receptors
- PMID: 18832151
- PMCID: PMC2563095
- DOI: 10.1073/pnas.0805590105
Evolution of vertebrate opioid receptors
Abstract
The opioid peptides and receptors have prominent roles in pain transmission and reward mechanisms in mammals. The evolution of the opioid receptors has so far been little studied, with only a few reports on species other than tetrapods. We have investigated species representing a broader range of vertebrates and found that the four opioid receptor types (delta, kappa, mu, and NOP) are present in most of the species. The gene relationships were deduced by using both phylogenetic analyses and chromosomal location relative to 20 neighboring gene families in databases of assembled genomes. The combined results show that the vertebrate opioid receptor gene family arose by quadruplication of a large chromosomal block containing at least 14 other gene families. The quadruplication seems to coincide with, and, therefore, probably resulted from, the two proposed genome duplications in early vertebrate evolution. We conclude that the quartet of opioid receptors was already present at the origin of jawed vertebrates approximately 450 million years ago. A few additional opioid receptor gene duplications have occurred in bony fishes. Interestingly, the ancestral receptor gene duplications coincide with the origin of the four opioid peptide precursor genes. Thus, the complete vertebrate opioid system was already established in the first jawed vertebrates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
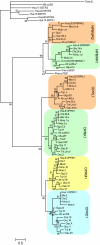


References
-
- Pert CB, Snyder SH. Opiate receptor: Demonstration in nervous tissue. Science. 1973;179:1011–1014. - PubMed
-
- Terenius L. Stereospecific interaction between narcotic analgesics and a synaptic plasma membrane fraction of rat cerebral cortex. Acta Pharmacol Toxicol (Copenhagen) 1973;32:317–320. - PubMed
-
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. - PubMed
-
- Evans CJ, Keith DE, Jr, Morrison H, Magendzo K, Edwards RH. Cloning of a delta opioid receptor by functional expression. Science. 1992;258:1952–1955. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

