The control of Ca2+ influx and NFATc3 signaling in arterial smooth muscle during hypertension
- PMID: 18832165
- PMCID: PMC2557027
- DOI: 10.1073/pnas.0808759105
The control of Ca2+ influx and NFATc3 signaling in arterial smooth muscle during hypertension
Abstract
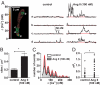
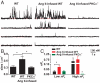
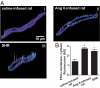
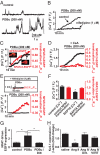
Many excitable cells express L-type Ca(2+) channels (LTCCs), which participate in physiological and pathophysiological processes ranging from memory, secretion, and contraction to epilepsy, heart failure, and hypertension. Clusters of LTCCs can operate in a PKCalpha-dependent, high open probability mode that generates sites of sustained Ca(2+) influx called "persistent Ca(2+) sparklets." Although increased LTCC activity is necessary for the development of vascular dysfunction during hypertension, the mechanisms leading to increased LTCC function are unclear. Here, we tested the hypothesis that increased PKCalpha and persistent Ca(2+) sparklet activity contributes to arterial dysfunction during hypertension. We found that PKCalpha and persistent Ca(2+) sparklet activity is indeed increased in arterial myocytes during hypertension. Furthermore, in human arterial myocytes, PKCalpha-dependent persistent Ca(2+) sparklets activated the prohypertensive calcineurin/NFATc3 signaling cascade. These events culminated in three hallmark signs of hypertension-associated vascular dysfunction: increased Ca(2+) entry, elevated arterial [Ca(2+)](i), and enhanced myogenic tone. Consistent with these observations, we show that PKCalpha ablation is protective against the development of angiotensin II-induced hypertension. These data support a model in which persistent Ca(2+) sparklets, PKCalpha, and calcineurin form a subcellular signaling triad controlling NFATc3-dependent gene expression, arterial function, and blood pressure. Because of the ubiquity of these proteins, this model may represent a general signaling pathway controlling gene expression and cellular function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: Prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774. - PubMed
-
- Rusch NJ, Hermsmeyer K. Calcium currents are altered in the vascular muscle cell membrane of spontaneously hypertensive rats. Circ Res. 1988;63:997–1002. - PubMed
-
- Ohya Y, Abe I, Fujii K, Takata Y, Fujishima M. Voltage-dependent Ca2+ channels in resistance arteries from spontaneously hypertensive rats. Circ Res. 1993;73:1090–1099. - PubMed
-
- Wang SQ, Song LS, Lakatta EG, Cheng H. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 2001;410:592–596. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

