Maraviroc for previously treated patients with R5 HIV-1 infection
- PMID: 18832244
- PMCID: PMC3078519
- DOI: 10.1056/NEJMoa0803152
Maraviroc for previously treated patients with R5 HIV-1 infection
Abstract
Background: CC chemokine receptor 5 antagonists are a new class of antiretroviral agents.
Methods: We conducted two double-blind, placebo-controlled, phase 3 studies--Maraviroc versus Optimized Therapy in Viremic Antiretroviral Treatment-Experienced Patients (MOTIVATE) 1 and MOTIVATE 2--with patients who had R5 human immunodeficiency virus type 1 (HIV-1) only. They had been treated with or had resistance to three antiretroviral-drug classes and had HIV-1 RNA levels of more than 5000 copies per milliliter. The patients were randomly assigned to one of three antiretroviral regimens consisting of maraviroc once daily, maraviroc twice daily, or placebo, each of which included optimized background therapy (OBT) based on treatment history and drug-resistance testing. Safety and efficacy were assessed after 48 weeks.
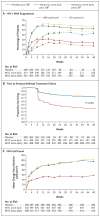
Results: A total of 1049 patients received the randomly assigned study drug; the mean baseline HIV-1 RNA level was 72,400 copies per milliliter, and the median CD4 cell count was 169 per cubic millimeter. At 48 weeks, in both studies, the mean change in HIV-1 RNA from baseline was greater with maraviroc than with placebo: -1.66 and -1.82 log(10) copies per milliliter with the once-daily and twice-daily regimens, respectively, versus -0.80 with placebo in MOTIVATE 1, and -1.72 and -1.87 log(10) copies per milliliter, respectively, versus -0.76 with placebo in MOTIVATE 2. More patients receiving maraviroc once or twice daily had HIV-1 RNA levels of less than 50 copies per milliliter (42% and 47%, respectively, vs. 16% in the placebo group in MOTIVATE 1; 45% in both maraviroc groups vs. 18% in MOTIVATE 2; P<0.001 for both comparisons in each study). The change from baseline in CD4 counts was also greater with maraviroc once or twice daily than with placebo (increases of 113 and 122 per cubic millimeter, respectively, vs. 54 in MOTIVATE 1; increases of 122 and 128 per cubic millimeter, respectively, vs. 69 in MOTIVATE 2; P<0.001 for both comparisons in each study). Frequencies of adverse events were similar among the groups.
Conclusions: Maraviroc, as compared with placebo, resulted in significantly greater suppression of HIV-1 and greater increases in CD4 cell counts at 48 weeks in previously treated patients with R5 HIV-1 who were receiving OBT. (ClinicalTrials.gov numbers, NCT00098306 and NCT00098722.)
2008 Massachusetts Medical Society
Figures

Comment in
-
A new class of anti-HIV therapy and new challenges.N Engl J Med. 2008 Oct 2;359(14):1509-11. doi: 10.1056/NEJMe0806598. N Engl J Med. 2008. PMID: 18832250 No abstract available.
-
Lessons from maraviroc clinical trials.Expert Rev Anti Infect Ther. 2011 Jun;9(6):649-51. doi: 10.1586/eri.11.52. Expert Rev Anti Infect Ther. 2011. PMID: 21692669 Review.
References
-
- AIDSinfo. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Rockville, MD: Department of Health and Human Services; Oct 102006. [Accessed September 9, 2008]. http://aidsinfo.nih.gov/Guidelines/ArchivedGuidelines.aspx.
-
- Phillips AN, Dunn D, Sabin C, et al. Long term probability of detection of HIV-1 drug resistance after starting anti-retroviral therapy in routine clinical practice. AIDS. 2005;19:487–94. - PubMed
-
- Mocroft A, Devereux H, Kinloch-de-Loes S, et al. Immunological, virological and clinical response to highly active anti-retroviral therapy treatment regimens in a complete clinic population. AIDS. 2000;14:1545–52. - PubMed
-
- AIDSinfo. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Rockville, MD: Department of Health and Human Services; Jan 292008. [Accessed September 9, 2008]. http://aidsinfo.nih.gov/guidelines.
-
- Liu R, Paxton WA, Choe S, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–77. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
