Distinct expressions of contrast gain control in parallel synaptic pathways converging on a retinal ganglion cell
- PMID: 18832424
- PMCID: PMC2655385
- DOI: 10.1113/jphysiol.2008.156224
Distinct expressions of contrast gain control in parallel synaptic pathways converging on a retinal ganglion cell
Abstract
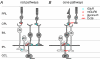
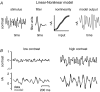
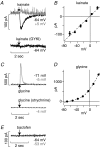
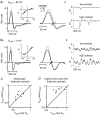
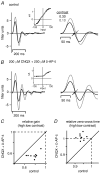
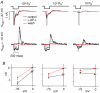
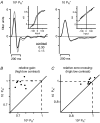
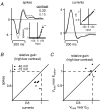
Visual neurons adapt to increases in stimulus contrast by reducing their response sensitivity and decreasing their integration time, a collective process known as 'contrast gain control.' In retinal ganglion cells, gain control arises at two stages: an intrinsic mechanism related to spike generation, and a synaptic mechanism in retinal pathways. Here, we tested whether gain control is expressed similarly by three synaptic pathways that converge on an OFF alpha/Y-type ganglion cell: excitatory inputs driven by OFF cone bipolar cells; inhibitory inputs driven by ON cone bipolar cells; and inhibitory inputs driven by rod bipolar cells. We made whole-cell recordings of membrane current in guinea pig ganglion cells in vitro. At high contrast, OFF bipolar cell-mediated excitatory input reduced gain and shortened integration time. Inhibitory input was measured by clamping voltage near 0 mV or by recording in the presence of ionotropic glutamate receptor (iGluR) antagonists to isolate the following circuit: cone --> ON cone bipolar cell --> AII amacrine cell --> OFF ganglion cell. At high contrast, this input reduced gain with no effect on integration time. Mean luminance was reduced 1000-fold to recruit the rod bipolar pathway: rod --> rod bipolar cell --> AII cell --> OFF ganglion cell. The spiking response, measured with loose-patch recording, adapted despite essentially no gain control in synaptic currents. Thus, cone bipolar-driven pathways adapt differently, with kinetic effects confined to the excitatory OFF pathway. The ON bipolar-mediated inhibition reduced gain at high contrast by a mechanism that did not require an iGluR. Under rod bipolar-driven conditions, ganglion cell firing showed gain control that was explained primarily by an intrinsic property.
Figures
References
-
- Baccus SA, Meister M. Fast and slow contrast adaptation in retinal circuitry. Neuron. 2002;36:909–919. - PubMed
-
- Benardete EA, Kaplan E. The dynamics of primate M retinal ganglion cells. Vis Neurosci. 1999;16:355–368. - PubMed
-
- Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res. 2001;20:351–384. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

