mda-9/Syntenin promotes metastasis in human melanoma cells by activating c-Src
- PMID: 18832467
- PMCID: PMC2572986
- DOI: 10.1073/pnas.0808171105
mda-9/Syntenin promotes metastasis in human melanoma cells by activating c-Src
Abstract
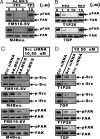
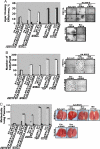
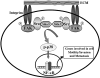
The scaffold PDZ-domain containing protein mda-9/syntenin functions as a positive regulator of cancer cell progression in human melanoma and other tumors. mda-9/Syntenin regulates cell motility and invasion by altering defined biochemical and signaling pathways, including focal adhesion kinase (FAK), p38 mitogen-activated protein kinase (MAPK) and NF-kappaB, but precisely how mda-9/syntenin organizes these multiprotein signaling complexes is not well understood. Using a clinically relevant human melanoma model, we demonstrate that mda-9/syntenin physically interacts with c-Src and this communication correlates with an increase in FAK/c-Src complex formation and c-Src activation. Inhibiting mda-9/syntenin, using an adenovirus expressing antisense mda-9/syntenin or addition of c-Src siRNA, suppresses melanoma cell migration, anchorage-independent growth, and spontaneous tumor cell dissemination in vivo in a human melanoma animal metastasis model. These data are compatible with a model wherein interaction of MDA-9/syntenin with c-Src promotes the formation of an active FAK/c-Src signaling complex, leading to enhanced tumor cell invasion and metastatic spread. These provocative findings highlight mda-9/syntenin and its interacting partners as promising therapeutic targets for intervention of metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Shah GD, Chapman PB. Adjuvant therapy of melanoma. Cancer J. 2007;13:217–222. - PubMed
-
- Lin JJ, Jiang H, Fisher PB. Characterization of a novel melanoma differentiation-associated gene, mda-9, that is down regulated during terminal differentiation. Mol Cell Differ. 1996;4:317–333.
-
- Lin JJ, Jiang H, Fisher PB. Melanoma differentiation associated gene-9, mda-9, is a human gamma interferon responsive gene. Gene. 1998;207:105–110. - PubMed
-
- Boukerche H, et al. mda-9/Syntenin: A positive regulator of melanoma metastasis. Cancer Res. 2005;65:10901–10911. - PubMed
-
- Helmke BM, et al. Melanoma metastasis is associated with enhanced expression of the syntenin gene. Oncol Rep. 2004;12:221–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

