Once daily versus three times daily mesalazine granules in active ulcerative colitis: a double-blind, double-dummy, randomised, non-inferiority trial
- PMID: 18832520
- PMCID: PMC3269751
- DOI: 10.1136/gut.2008.154302
Once daily versus three times daily mesalazine granules in active ulcerative colitis: a double-blind, double-dummy, randomised, non-inferiority trial
Abstract
Objectives: To determine the therapeutic equivalence and safety of once daily (OD) versus three times daily (TID) dosing of a total daily dose of 3 g Salofalk (mesalazine) granules in patients with active ulcerative colitis.
Design: A randomised, double-blind, double-dummy, parallel group, multicentre, international, phase III non-inferiority study.
Setting: 54 centres in 13 countries.
Patients: 380 patients with confirmed diagnosis of established or first attack of ulcerative colitis (clinical activity index (CAI)>4 and endoscopic index > or =4 at baseline) were randomised and treated.
Interventions: 8-week treatment with either 3 g OD or 1 g TID mesalazine granules.
Main outcome measures: Clinical remission (CAI< or =4) at study end.
Results: 380 patients were evaluable for efficacy and safety by intention-to-treat (ITT); 345 for per protocol (PP) analysis. In the ITT population, 79.1% in the OD group (n = 191) and 75.7% in the TID group (n = 189) achieved clinical remission (p<0.0001 for non-inferiority). Significantly more patients with proctosigmoiditis achieved clinical remission in the OD group (86%; n = 97) versus the TID group (73%; n = 100; p = 0.0298). About 70% of patients in both treatment groups achieved endoscopic remission, and 35% in the OD group and 41% in the TID group achieved histological remission. About 80% of all patients preferred OD dosing. Similar numbers of adverse events occurred in 55 patients (28.8%) in the OD group and in 61 patients (32.3%) in the TID group, indicating that the two dosing regimens were equally safe and well tolerated.
Conclusions: OD 3 g mesalazine granules are as effective and safe as a TID 1 g schedule. With respect to the best possible adherence of patients to the treatment, OD dosing of mesalazine should be the preferred application mode in active ulcerative colitis.
Trial registration: ClinicalTrials.gov NCT00449722.
Conflict of interest statement
Figures


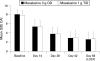
References
-
- Ardizzone S, Porro GB. A practical guide to the management of distal ulcerative colitis. Drugs 1998;55:519–42 - PubMed
-
- Hanauer SB. Medical therapy for ulcerative colitis 2004 [review]. Gastroenterology 2004;126:1582–92 - PubMed
-
- Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 2004;99:1371–85 - PubMed
-
- Allgayer H, Kruis W. Aminosalicylates: Potential antineoplastic actions in colon cancer prevention. Scand J Gastroenterol 2002;37:125–31 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
