Overexpression of mIGF-1 in keratinocytes improves wound healing and accelerates hair follicle formation and cycling in mice
- PMID: 18832567
- PMCID: PMC2570121
- DOI: 10.2353/ajpath.2008.071177
Overexpression of mIGF-1 in keratinocytes improves wound healing and accelerates hair follicle formation and cycling in mice
Abstract
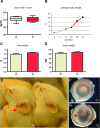
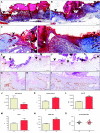
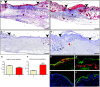
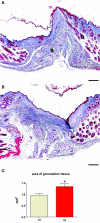
Insulin-like growth factor 1 (IGF-1) is an important regulator of growth, survival, and differentiation in many tissues. It is produced in several isoforms that differ in their N-terminal signal peptide and C-terminal extension peptide. The locally acting isoform of IGF-1 (mIGF-1) was previously shown to enhance the regeneration of both muscle and heart. In this study, we tested the therapeutic potential of mIGF-1 in the skin by generating a transgenic mouse model in which mIGF-1 expression is driven by the keratin 14 promoter. IGF-1 levels were unchanged in the sera of hemizygous K14/mIGF-1 transgenic animals whose growth was unaffected. A skin analysis of young animals revealed normal architecture and thickness as well as proper expression of differentiation and proliferation markers. No malignant tumors were formed. Normal homeostasis of the putative stem cell compartment was also maintained. Healing of full-thickness excisional wounds was accelerated because of increased proliferation and migration of keratinocytes, whereas inflammation, granulation tissue formation, and scarring were not obviously affected. In addition, mIGF-1 promoted late hair follicle morphogenesis and cycling. To our knowledge, this is the first work to characterize the simultaneous, stimulatory effect of IGF-1 delivery to keratinocytes on two types of regeneration processes within a single mouse model. Our analysis supports the use of mIGF-1 for skin and hair regeneration and describes a potential cell type-restricted action.
Figures




References
-
- Dupont J, Holzenberger M. Biology of insulin-like growth factors in development, Birth Defects Res C Embryo Today. 2003;69:257–271. - PubMed
-
- Edmondson SR, Thumiger SP, Werther GA, Wraight CJ. Epidermal homeostasis: the role of the growth hormone and insulin-like growth factor systems. Endocr Rev. 2003;24:737–764. - PubMed
-
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 1993;75:59–72. - PubMed
-
- Lange M, Thulesen J, Feldt-Rasmussen U, Skakkebaek NE, Vahl N, Jorgensen JO, Christiansen JS, Poulsen SS, Sneppen SB, Juul A. Skin morphological changes in growth hormone deficiency and acromegaly. Eur J Endocrinol. 2001;145:147–153. - PubMed
-
- Lurie R, Ben-Amitai D, Laron Z. Laron syndrome (primary growth hormone insensitivity): a unique model to explore the effect of insulin-like growth factor 1 deficiency on human hair. Dermatology. 2004;208:314–318. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

