Focal adhesion kinase-related proline-rich tyrosine kinase 2 and focal adhesion kinase are co-overexpressed in early-stage and invasive ErbB-2-positive breast cancer and cooperate for breast cancer cell tumorigenesis and invasiveness
- PMID: 18832579
- PMCID: PMC2570143
- DOI: 10.2353/ajpath.2008.080292
Focal adhesion kinase-related proline-rich tyrosine kinase 2 and focal adhesion kinase are co-overexpressed in early-stage and invasive ErbB-2-positive breast cancer and cooperate for breast cancer cell tumorigenesis and invasiveness
Abstract
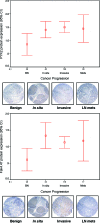
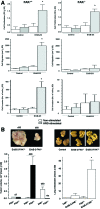
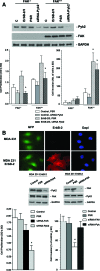
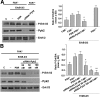
Early cancer cell migration and invasion of neighboring tissues are mediated by multiple events, including activation of focal adhesion signaling. Key regulators include the focal adhesion kinase (FAK) and FAK-related proline-rich tyrosine kinase 2 (Pyk2), whose distinct functions in cancer progression remain unclear. Here, we compared Pyk2 and FAK expression in breast cancer and their effects on ErbB-2-induced tumorigenesis and the potential therapeutic utility of targeting Pyk2 compared with FAK in preclinical models of breast cancer. Pyk2 is overexpressed in tissues from early and advanced breast cancers and overexpressed with both FAK and epidermal growth factor receptor-2 (ErbB-2) in a subset of breast cancer cases. Down-regulation of Pyk2 in ErbB-2-positive, FAK-proficient, and FAK-deficient cells reduced cell proliferation, which correlated with reduced mitogen-activated protein kinase (MAPK) activity. In contrast, Pyk2 silencing had little impact on cell migration and invasion. In vivo, Pyk2 down-regulation reduced primary tumor growth induced by a metastatic variant of ErbB-2-positive MDA 231 breast cancer cells but had little effect on lung metastases in contrast to FAK down-regulation. Dual reduction of Pyk2 and FAK expression resulted in strong inhibition of both primary tumor growth and lung metastases. Together, these data support the cooperative function of Pyk2 and FAK in breast cancer progression and suggest that dual inhibition of FAK and Pyk2 is an efficient therapeutic approach for targeting invasive breast cancer.
Figures

References
-
- Du QS, Ren XR, Xie Y, Wang Q, Mei L, Xiong WC. Inhibition of PYK2-induced actin cytoskeleton reorganization: PYK2 autophosphorylation and focal adhesion targeting by FAK. J Cell Sci. 2001;114:2977–2987. - PubMed
-
- Chen HC, Appeddu PA, Parsons JT, Hildebrand JD, Schaller MD, Guan JL. Interaction of focal adhesion kinase with cytoskeletal protein talin. J Biol Chem. 1995;270:16995–16999. - PubMed
-
- Vadlamudi RK, Adam L, Nguyen D, Santos M, Kumar R. Differential regulation of components of the focal adhesion complex by heregulin: role of phosphatase SHP-2. J Cell Physiol. 2002;190:189–199. - PubMed
-
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. - PubMed
-
- Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–256. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

