PD-1-dependent mechanisms maintain peripheral tolerance of donor-reactive CD8+ T cells to transplanted tissue
- PMID: 18832687
- PMCID: PMC2572818
- DOI: 10.4049/jimmunol.181.8.5313
PD-1-dependent mechanisms maintain peripheral tolerance of donor-reactive CD8+ T cells to transplanted tissue
Abstract
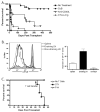
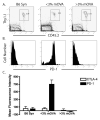
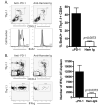
Peripheral mechanisms of self-tolerance often depend on the quiescent state of the immune system. To what degree such mechanisms can be engaged in the enhancement of allograft survival is unclear. To examine the role of the PD-1 pathway in the maintenance of graft survival following blockade of costimulatory pathways, we used a single-Ag mismatch model of graft rejection where we could track the donor-specific cells as they developed endogenously and emerged from the thymus. We found that graft-specific T cells arising under physiologic developmental conditions at low frequency were actively deleted at the time of transplantation under combined CD28/CD40L blockade. However, this deletion was incomplete, and donor-specific cells that failed to undergo deletion up-regulated expression of PD-1. Furthermore, blockade of PD-1 signaling on these cells via in vivo treatment with anti-PD-1 mAb resulted in rapid expansion of donor-specific T cells and graft loss. These results suggest that the PD-1 pathway was engaged in the continued regulation of the low-frequency graft-specific immune response and thus in maintenance of graft survival.
Figures


References
-
- Jenkins MK. The ups and downs of T cell costimulation. Immunity. 1994;1:443–446. - PubMed
-
- Pearson TC, Alexander DZ, Corbascio M, Hendrix R, Ritchie SC, Linsley PS, Faherty D, Larsen CP. Analysis of the B7 costimulatory pathway in allograft rejection. Transplantation. 1997;63:1463–1469. - PubMed
-
- Pearson TC, Alexander DZ, Winn KJ, Linsley PS, Lowry RP, Larsen CP. Transplantation tolerance induced by CTLA4-Ig. Transplantation. 1994;57:1701–1706. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

