Ca2+ influx through T- and L-type Ca2+ channels have different effects on myocyte contractility and induce unique cardiac phenotypes
- PMID: 18832749
- PMCID: PMC2678411
- DOI: 10.1161/CIRCRESAHA.108.185611
Ca2+ influx through T- and L-type Ca2+ channels have different effects on myocyte contractility and induce unique cardiac phenotypes
Abstract
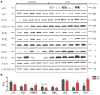
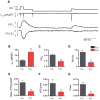
T-type Ca(2+) channels (TTCCs) are expressed in the developing heart, are not present in the adult ventricle, and are reexpressed in cardiac diseases involving cardiac dysfunction and premature, arrhythmogenic death. The goal of this study was to determine the functional role of increased Ca(2+) influx through reexpressed TTCCs in the adult heart. A mouse line with cardiac-specific, conditional expression of the alpha1G-TTCC was used to increase Ca(2+) influx through TTCCs. alpha1G hearts had mild increases in contractility but no cardiac histopathology or premature death. This contrasts with the pathological phenotype of a previously studied mouse with increased Ca(2+) influx through the L-type Ca(2+) channel (LTCC) secondary to overexpression of its beta2a subunit. Although alpha1G and beta2a myocytes had similar increases in Ca(2+) influx, alpha1G myocytes had smaller increases in contraction magnitude, and, unlike beta2a myocytes, there were no increases in sarcoplasmic reticulum Ca(2+) loading. Ca(2+) influx through TTCCs also did not induce normal sarcoplasmic reticulum Ca(2+) release. alpha1G myocytes had changes in LTCC, SERCA2a, and phospholamban abundance, which appear to be adaptations that help maintain Ca(2+) homeostasis. Immunostaining suggested that the majority of alpha1G-TTCCs were on the surface membrane. Osmotic shock, which selectively eliminates T-tubules, induced a greater reduction in L- versus TTCC currents. These studies suggest that T- and LTCCs are in different portions of the sarcolemma (surface membrane versus T-tubules) and that Ca(2+) influx through these channels induce different effects on myocyte contractility and lead to distinct cardiac phenotypes.
Figures






References
-
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. - PubMed
-
- Bers DM, Perez-Reyes E. Ca channels in cardiac myocytes: structure and function in Ca influx and intracellular Ca release. Cardiovasc Res. 1999;42:339–360. - PubMed
-
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. - PubMed
-
- Frey N, McKinsey TA, Olson EN. Decoding calcium signals involved in cardiac growth and function. Nat Med. 2000;6:1221–1227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

