A novel disulphide switch mechanism in Ero1alpha balances ER oxidation in human cells
- PMID: 18833192
- PMCID: PMC2585162
- DOI: 10.1038/emboj.2008.202
A novel disulphide switch mechanism in Ero1alpha balances ER oxidation in human cells
Abstract
Oxidative maturation of secretory and membrane proteins in the endoplasmic reticulum (ER) is powered by Ero1 oxidases. To prevent cellular hyperoxidation, Ero1 activity can be regulated by intramolecular disulphide switches. Here, we determine the redox-driven shutdown mechanism of Ero1alpha, the housekeeping Ero1 enzyme in human cells. We show that functional silencing of Ero1alpha in cells arises from the formation of a disulphide bond-identified by mass spectrometry--between the active-site Cys(94) (connected to Cys(99) in the active enzyme) and Cys(131). Competition between substrate thiols and Cys(131) creates a feedback loop where activation of Ero1alpha is linked to the availability of its substrate, reduced protein disulphide isomerase (PDI). Overexpression of Ero1alpha-Cys131Ala or the isoform Ero1beta, which does not have an equivalent disulphide switch, leads to augmented ER oxidation. These data reveal a novel regulatory feedback system where PDI emerges as a central regulator of ER redox homoeostasis.
Figures

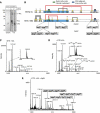
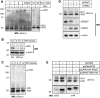


Comment in
-
A non-catalytic disulphide bond regulating redox flux in the ER oxidative folding pathway.EMBO J. 2009 Feb 4;28(3):169-70. doi: 10.1038/emboj.2008.293. EMBO J. 2009. PMID: 19194483 Free PMC article. No abstract available.
References
-
- Appenzeller-Herzog C, Ellgaard L (2008a) In vivo reduction–oxidation state of protein disulfide isomerase: the two active sites independently occur in the reduced and oxidized forms. Antioxid Redox Signal 10: 55–64 - PubMed
-
- Appenzeller-Herzog C, Ellgaard L (2008b) The human PDI family: versatility packed into a single fold. Biochim Biophys Acta 1783: 535–548 - PubMed
-
- Bertoli G, Simmen T, Anelli T, Molteni SN, Fesce R, Sitia R (2004) Two conserved cysteine triads in human Ero1alpha cooperate for efficient disulfide bond formation in the endoplasmic reticulum. J Biol Chem 279: 30047–30052 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

