The role of Gbetagamma subunits in the organization, assembly, and function of GPCR signaling complexes
- PMID: 18834311
- PMCID: PMC2659589
- DOI: 10.1146/annurev-pharmtox-061008-103038
The role of Gbetagamma subunits in the organization, assembly, and function of GPCR signaling complexes
Abstract
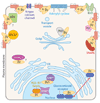
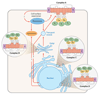
The role of Gbetagamma subunits in cellular signaling has become well established in the past 20 years. Not only do they regulate effectors once thought to be the sole targets of Galpha subunits, but it has become clear that they also have a unique set of binding partners and regulate signaling pathways that are not always localized to the plasma membrane. However, this may be only the beginning of the story. Gbetagamma subunits interact with G protein-coupled receptors, Galpha subunits, and several different effector molecules during assembly and trafficking of receptor-based signaling complexes and not simply in response to ligand stimulation at sites of receptor cellular activity. Gbetagamma assembly itself seems to be tightly regulated via the action of molecular chaperones and in turn may serve a similar role in the assembly of specific signaling complexes. We propose that specific Gbetagamma subunits have a broader role in controlling the architecture, assembly, and activity of cellular signaling pathways.
Figures

References
-
- Gether U, Kobilka BK. G protein-coupled receptors. II. Mechanism of agonist activation. J. Biol. Chem. 1998;273:17979–17982. - PubMed
-
- Lagerstrom MC, Schioth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 2008;7:339–357. - PubMed
-
- Shenker A, Laue L, Kosugi S, Merendino JJ, Jr, Minegishi T, Cutler GB., Jr A constitutively activating mutation of the luteinizing hormone receptor in familial male precocious puberty. Nature. 1993;365:652–654. - PubMed
-
- Robinson PR, Cohen GB, Zhukovsky EA, Oprian DD. Constitutively active mutants of rhodopsin. Neuron. 1992;9:719–725. - PubMed
-
- Parma J, Dupréz L, Van Sande J, Cochaux P, Gervy C, et al. Somatic mutations in the thyrotropin receptor gene cause hyperfunctioning thyroid adenomas. Nature. 1993;365:649–651. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

