Parametric study of EEG sensitivity to phase noise during face processing
- PMID: 18834518
- PMCID: PMC2573889
- DOI: 10.1186/1471-2202-9-98
Parametric study of EEG sensitivity to phase noise during face processing
Abstract
Background: The present paper examines the visual processing speed of complex objects, here faces, by mapping the relationship between object physical properties and single-trial brain responses. Measuring visual processing speed is challenging because uncontrolled physical differences that co-vary with object categories might affect brain measurements, thus biasing our speed estimates. Recently, we demonstrated that early event-related potential (ERP) differences between faces and objects are preserved even when images differ only in phase information, and amplitude spectra are equated across image categories. Here, we use a parametric design to study how early ERP to faces are shaped by phase information. Subjects performed a two-alternative force choice discrimination between two faces (Experiment 1) or textures (two control experiments). All stimuli had the same amplitude spectrum and were presented at 11 phase noise levels, varying from 0% to 100% in 10% increments, using a linear phase interpolation technique. Single-trial ERP data from each subject were analysed using a multiple linear regression model.
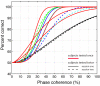
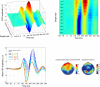
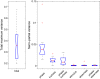
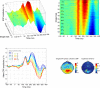
Results: Our results show that sensitivity to phase noise in faces emerges progressively in a short time window between the P1 and the N170 ERP visual components. The sensitivity to phase noise starts at about 120-130 ms after stimulus onset and continues for another 25-40 ms. This result was robust both within and across subjects. A control experiment using pink noise textures, which had the same second-order statistics as the faces used in Experiment 1, demonstrated that the sensitivity to phase noise observed for faces cannot be explained by the presence of global image structure alone. A second control experiment used wavelet textures that were matched to the face stimuli in terms of second- and higher-order image statistics. Results from this experiment suggest that higher-order statistics of faces are necessary but not sufficient to obtain the sensitivity to phase noise function observed in response to faces.
Conclusion: Our results constitute the first quantitative assessment of the time course of phase information processing by the human visual brain. We interpret our results in a framework that focuses on image statistics and single-trial analyses.
Figures











Similar articles
-
Early ERPs to faces and objects are driven by phase, not amplitude spectrum information: evidence from parametric, test-retest, single-subject analyses.J Vis. 2012 Dec 14;12(13):12. doi: 10.1167/12.13.12. J Vis. 2012. PMID: 23241265
-
Single-trial EEG dynamics of object and face visual processing.Neuroimage. 2007 Jul 1;36(3):843-62. doi: 10.1016/j.neuroimage.2007.02.052. Epub 2007 Mar 23. Neuroimage. 2007. PMID: 17475510
-
The N170, not the P1, indexes the earliest time for categorical perception of faces, regardless of interstimulus variance.Neuroimage. 2012 Sep;62(3):1563-74. doi: 10.1016/j.neuroimage.2012.05.043. Epub 2012 May 24. Neuroimage. 2012. PMID: 22634853
-
Does physical interstimulus variance account for early electrophysiological face sensitive responses in the human brain? Ten lessons on the N170.Neuroimage. 2008 Feb 15;39(4):1959-79. doi: 10.1016/j.neuroimage.2007.10.011. Epub 2007 Oct 22. Neuroimage. 2008. PMID: 18055223 Review.
-
Understanding face perception by means of human electrophysiology.Trends Cogn Sci. 2014 Jun;18(6):310-8. doi: 10.1016/j.tics.2014.02.013. Epub 2014 Apr 1. Trends Cogn Sci. 2014. PMID: 24703600 Review.
Cited by
-
LIMO EEG: a toolbox for hierarchical LInear MOdeling of ElectroEncephaloGraphic data.Comput Intell Neurosci. 2011;2011:831409. doi: 10.1155/2011/831409. Epub 2011 Feb 21. Comput Intell Neurosci. 2011. PMID: 21403915 Free PMC article.
-
It's about Time.Front Hum Neurosci. 2011 Jan 19;5:2. doi: 10.3389/fnhum.2011.00002. eCollection 2011. Front Hum Neurosci. 2011. PMID: 21267395 Free PMC article.
-
The background of reduced face specificity of N170 in congenital prosopagnosia.PLoS One. 2014 Jul 1;9(7):e101393. doi: 10.1371/journal.pone.0101393. eCollection 2014. PLoS One. 2014. PMID: 24983881 Free PMC article.
-
Stimulus dependency of object-evoked responses in human visual cortex: an inverse problem for category specificity.PLoS One. 2012;7(2):e30727. doi: 10.1371/journal.pone.0030727. Epub 2012 Feb 17. PLoS One. 2012. PMID: 22363479 Free PMC article.
-
Dissociating the effect of noise on sensory processing and overall decision difficulty.J Neurosci. 2011 Feb 16;31(7):2663-74. doi: 10.1523/JNEUROSCI.2725-10.2011. J Neurosci. 2011. PMID: 21325535 Free PMC article.
References
-
- Bullier J, Hupe JM, James AC, Girard P. The role of feedback connections in shaping the responses of visual cortical neurons. Prog Brain Res. 2001;134:193–204. - PubMed
-
- Bullier J. Integrated model of visual processing. Brain Research Reviews. 2001;36(2–3):96–107. - PubMed
-
- DiCarlo JJ, Cox DD. Untangling invariant object recognition. Trends Cogn Sci. 2007;11(8):333–41. - PubMed
-
- Rust NC, Movshon JA. In praise of artifice. Nat Neurosci. 2005;8(12):1647–50. - PubMed
-
- Felsen G, Dan Y. A natural approach to studying vision. Nat Neurosci. 2005;8(12):1643–6. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

