Plasminogen activator inhibitor-1 protects endothelial cells from FasL-mediated apoptosis
- PMID: 18835034
- PMCID: PMC2630529
- DOI: 10.1016/j.ccr.2008.08.012
Plasminogen activator inhibitor-1 protects endothelial cells from FasL-mediated apoptosis
Abstract
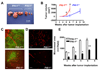
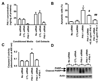
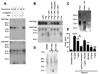
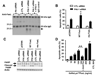
Plasminogen activator inhibitor-1 (PAI-1) paradoxically enhances tumor progression and angiogenesis; however, the mechanism supporting this role is not known. Here we provide evidence that PAI-1 is essential to protect endothelial cells (ECs) from FasL-mediated apoptosis. In the absence of host-derived PAI-1, human neuroblastoma cells implanted in PAI-1-deficient mice form smaller and poorly vascularized tumors containing an increased number of apoptotic ECs. We observed that knockdown of PAI-1 in ECs enhances cell-associated plasmin activity and increases spontaneous apoptosis in vitro. We further demonstrate that plasmin cleaves FasL at Arg144-Lys145, releasing a soluble proapoptotic FasL fragment from the surface of ECs. The data provide a mechanism explaining the proangiogenic activity of PAI-1.
Figures



References
-
- Aoudjit F, Vuori K. Engagement of the alpha2beta1 integrin inhibits Fas ligand expression and activation-induced cell death in T cells in a focal adhesion kinase-dependent manner. Blood. 2000;95:2044–2051. - PubMed
-
- Bajou K, Maillard C, Jost M, Lijnen RH, Gils A, Declerck P, Carmeliet P, Foidart JM, Noel A. Host-derived plasminogen activator inhibitor-1 (PAI-1) concentration is critical for in vivo tumoral angiogenesis and growth. Oncogene. 2004;23:6986–6990. - PubMed
-
- Bajou K, Masson V, Gerard RD, Schmitt PM, Albert V, Praus M, Lund LR, Frandsen TL, Brunner N, Dano K, Fusenig NE, Weidle U, Carmeliet G, Loskutoff D, Collen D, Carmeliet P, Foidart JM, Noel A. The plasminogen activator inhibitor PAI-1 controls in vivo tumor vascularization by interaction with proteases, not vitronectin. Implications for antiangiogenic strategies. J. Cell Biol. 2001;152:777–784. - PMC - PubMed
-
- Bajou K, Noel A, Gerard RD, Masson V, Brunner N, Holst-Hansen C, Skobe M, Fusenig NE, Carmeliet P, Collen D, Foidart JM. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat. Med. 1998;4:923–928. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

