Ferritin for the clinician
- PMID: 18835072
- PMCID: PMC2717717
- DOI: 10.1016/j.blre.2008.08.001
Ferritin for the clinician
Abstract
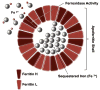
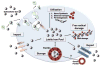
Ferritin, a major iron storage protein, is essential to iron homeostasis and is involved in a wide range of physiologic and pathologic processes. In clinical medicine, ferritin is predominantly utilized as a serum marker of total body iron stores. In cases of iron deficiency and overload, serum ferritin serves a critical role in both diagnosis and management. Elevated serum and tissue ferritin are linked to coronary artery disease, malignancy, and poor outcomes following stem cell transplantation. Ferritin is directly implicated in less common but potentially devastating human diseases including sideroblastic anemias, neurodegenerative disorders, and hemophagocytic syndrome. Additionally, recent research describes novel functions of ferritin independent of iron storage.
Figures

References
-
- Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol. 2007;69(69–85):69–85. - PubMed
-
- Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341(26):1986–1995. - PubMed
-
- Andrews NC. Understanding heme transport. N Engl J Med. 2005;353(23):2508–2509. - PubMed
-
- Anderson GJ, Frazer DM. Hepatic iron metabolism. Semin Liver Dis. 2005;25(4):420–432. - PubMed
-
- Heeney MM, Andrews NC. Iron homeostasis and inherited iron overload disorders: an overview. Hematol Oncol Clin North Am. 2004;18(6):1379–403. ix. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

