Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages: a potential mechanism for intercellular HIV trafficking
- PMID: 18835599
- PMCID: PMC2701345
- DOI: 10.1016/j.cellimm.2008.08.005
Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages: a potential mechanism for intercellular HIV trafficking
Abstract
Cell to cell communication is essential for the organization/coordination of multicellular systems and cellular development. Cellular communication is mediated by soluble factors, including growth factors, neurotransmitters, cytokines/chemokines, gap junctions, and the recently described tunneling nanotubes (TNT). TNT are long cytoplasmatic bridges that enable long range directed communication between cells. The proposed function for TNT is the cell-to-cell transfer of large cellular structures such as vesicles and organelles. We demonstrate that HIV-infection of human macrophages results in an increased number of TNT, and show HIV particles within these structures. We propose that HIV "highjacks" TNT communication to spread HIV through an intercellular route between communicated cells, contributing to the pathogenesis of AIDS.
Figures
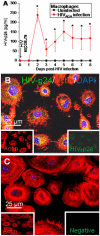

 , red line) macrophage cultures, ∼15-25% of the cells have filopodia. (B) HIV-infection of macrophages induces the formation of additional short TNT (
, red line) macrophage cultures, ∼15-25% of the cells have filopodia. (B) HIV-infection of macrophages induces the formation of additional short TNT ( , red line, *p < 0.001, n = 4) as compared to uninfected cultures of macrophages (■, black line). (C) HIV-infection of macrophages induces the formation of an increased number of long TNT (
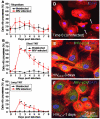
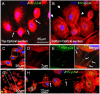
, red line, *p < 0.001, n = 4) as compared to uninfected cultures of macrophages (■, black line). (C) HIV-infection of macrophages induces the formation of an increased number of long TNT ( , red line, *p < 0.003, n = 4) as compared to uninfected cultures (■, black line). Statistical significance in (B and C) graphs, between uninfected and HIV-infected macrophages, was found at days 2-6 and 2-4, respectively, and returned to control levels (uninfected cells) after 5 and 6 days post-infection. (D) An example of filopodia at time 0 in uninfected cells stained for actin and DAPI. (E) An example of short range TNT, 3 days post-infection with HIV stained for HIV-p24 (green staining), actin (Texas Red-phalloidin, red staining) and DAPI (blue staining). (F) An example of a decrease in short and long TNT, 7 days post-infection. Arrow in (D) denotes the filopodia structures at time 0. Bar = 25 μm.
, red line, *p < 0.003, n = 4) as compared to uninfected cultures (■, black line). Statistical significance in (B and C) graphs, between uninfected and HIV-infected macrophages, was found at days 2-6 and 2-4, respectively, and returned to control levels (uninfected cells) after 5 and 6 days post-infection. (D) An example of filopodia at time 0 in uninfected cells stained for actin and DAPI. (E) An example of short range TNT, 3 days post-infection with HIV stained for HIV-p24 (green staining), actin (Texas Red-phalloidin, red staining) and DAPI (blue staining). (F) An example of a decrease in short and long TNT, 7 days post-infection. Arrow in (D) denotes the filopodia structures at time 0. Bar = 25 μm.
References
-
- Gerdes HH, Bukoreshtliev NV, Barroso JF. Tunneling nanotubes: a new route for the exchange of components between animal cells. FEBS Lett. 2007;581:2194–2201. - PubMed
-
- Onfelt B, Nedvetzki S, Yanagi K, Davis DM. Cutting edge: membrane nanotubes connect immune cells. J. Immunol. 2004;173:1511–1513. - PubMed
-
- Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. - PubMed
-
- Hsiung F, Ramirez-Weber FA, Iwaki DD, Kornberg TB. Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature. 2005;437:560–563. - PubMed
-
- Kornberg T. Pictures in cell biology. Cytonemes. Trends Cell Biol. 1999;9:434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

