Forward frontal fields: phylogeny and fundamental function
- PMID: 18835649
- PMCID: PMC2587508
- DOI: 10.1016/j.tins.2008.08.008
Forward frontal fields: phylogeny and fundamental function
Abstract
The largest part of the primate prefrontal cortex has no homolog in other mammals. Accordingly, it probably confers some advantage that other mammals either lack or attain through the function of other structures. Yet, this advantage remains enigmatic. This is not so for other parts of the cortex. For example, certain visual areas encode, represent and store knowledge about objects. By analogy, perhaps the primate prefrontal cortex encodes, represents and stores knowledge about behaviors, including the consequences of doing (or not doing) something in complex and challenging situations. The long list of functions often attributed to the prefrontal cortex could contribute to knowing what to do and what will happen when rare risks arise or outstanding opportunities knock.
Figures

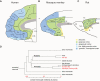
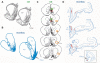

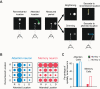
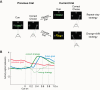
References
-
- Fulton JF. Physiology of the Nervous System. Oxford University Press; 1949.
-
- Wood JN, Grafman J. Human prefrontal cortex: Processing and representational perspectives. Nat. Rev. Neurosci. 2003;4:139–147. - PubMed
-
- Bussey TJ, et al. Impairments in visual discrimination after perirhinal cortex lesions: testing ‘declarative’ vs. ‘perceptual-mnemonic’ views of perirhinal cortex function. Eur. J. Neurosci. 2003;17:649–660. - PubMed
-
- Murray EA, et al. Visual perception and memory: A new view of medial temporal lobe function in primates and rodents. Annu. Rev. Neurosci. 2007;30:99–122. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

