Kinetic analysis of protein crystal nucleation in gel matrix
- PMID: 18835910
- PMCID: PMC2599815
- DOI: 10.1529/biophysj.108.135574
Kinetic analysis of protein crystal nucleation in gel matrix
Abstract
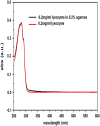
The effect of agarose on nucleation of hen egg white lysozyme crystal was examined quantitatively using a temperature-jumping technique. For the first time, to our knowledge, the inhibition of agarose during the nucleation of lysozyme was quantified in two respects: a), the effect of increasing interfacial nucleation barrier, described by the so-called interfacial correlation parameter f(m); and b), the ratio of diffusion to interfacial kinetics obtained from dynamic surface tension measurements. It follows from a dynamic surface tension analysis that the agarose network inhibits the nucleation of lysozyme by means of an enhancement of the repulsion and interfacial structure mismatch between foreign bodies and lysozyme crystals, slowing down the diffusion process of the protein molecules and clusters toward the crystal-fluid interface and inhibiting the rearrangement of protein molecules at the interface. Our results, based on ultraviolet-visible spectroscopy, also show no evidence of the supersaturation enhancement effect in protein agarose gels. The effects of nucleation suppression and transport limitation in gels result in bigger, fewer, and perhaps better quality protein crystals. The understandings obtained in this study will improve our knowledge in controlling the crystallization of proteins and other biomolecules.
Figures








Similar articles
-
Growth and characterization of high-quality protein crystals for X-ray crystallography.Ann N Y Acad Sci. 2009 Apr;1161:429-36. doi: 10.1111/j.1749-6632.2008.04078.x. Ann N Y Acad Sci. 2009. PMID: 19426336
-
Beneficial effect of solubility enhancers on protein crystal nucleation and growth.Langmuir. 2009 Apr 21;25(8):4579-87. doi: 10.1021/la803185m. Langmuir. 2009. PMID: 19309115
-
Crystal quality and differential crystal-growth behaviour of three proteins crystallized in gel at high hydrostatic pressure.Acta Crystallogr D Biol Crystallogr. 2005 Jun;61(Pt 6):784-8. doi: 10.1107/S0907444905007109. Epub 2005 May 26. Acta Crystallogr D Biol Crystallogr. 2005. PMID: 15930640
-
Recent developments in the kinetic theory of nucleation.Adv Colloid Interface Sci. 2005 Dec 30;118(1-3):51-72. doi: 10.1016/j.cis.2005.06.001. Epub 2005 Aug 30. Adv Colloid Interface Sci. 2005. PMID: 16137628 Review.
-
Protein crystallization.Annu Rev Phys Chem. 1996;47:171-204. doi: 10.1146/annurev.physchem.47.1.171. Annu Rev Phys Chem. 1996. PMID: 8983237 Review.
Cited by
-
Combining Surface Templating and Confinement for Controlling Pharmaceutical Crystallization.Pharmaceutics. 2020 Oct 20;12(10):995. doi: 10.3390/pharmaceutics12100995. Pharmaceutics. 2020. PMID: 33092148 Free PMC article. Review.
References
-
- McPherson, A. 1999. Crystallization of Biological Macromolecules. Cold Spring Harbor Press, Cold Spring Harbor, NY.
-
-
Matsuda, S., T. Senda, S. Itoh, G. Kawano, H. Mizuno, and Y. Mitsui. 1989. New crystal form of recombinant murine interferon-
J. Biol. Chem. 264:13381–13382. - PubMed
-
Matsuda, S., T. Senda, S. Itoh, G. Kawano, H. Mizuno, and Y. Mitsui. 1989. New crystal form of recombinant murine interferon-
-
- Hirsch, R. E., C. Raventos-Suarez, J. A. Olson, and R. L. Nagel. 1985. Ligand state of intraerythrocytic circulating HbC crystals in homozygote CC patients. Blood. 66:775–778. - PubMed
-
- Lorber, B., and R. Giegé. 2001. Nucleation and growth of thaumatin crystals within a gel under microgravity on STS-95 mission vs. under Earth's gravity. J. Cryst. Growth. 231:252–261.
-
- Moreno, A., B. Quiroz-García, F. Yokaichiya, V. Stojanoff, and P. Rudolph. 2007. Protein crystal growth in gels and stationary magnetic fields. Cryst. Res. Technol. 42:231–236.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

