Active Alu retrotransposons in the human genome
- PMID: 18836035
- PMCID: PMC2593586
- DOI: 10.1101/gr.081737.108
Active Alu retrotransposons in the human genome
Abstract
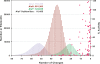
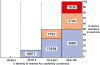
Alu retrotransposons evolved from 7SL RNA approximately 65 million years ago and underwent several rounds of massive expansion in primate genomes. Consequently, the human genome currently harbors 1.1 million Alu copies. Some of these copies remain actively mobile and continue to produce both genetic variation and diseases by "jumping" to new genomic locations. However, it is unclear how many active Alu copies exist in the human genome and which Alu subfamilies harbor such copies. Here, we present a comprehensive functional analysis of Alu copies across the human genome. We cloned Alu copies from a variety of genomic locations and tested these copies in a plasmid-based mobilization assay. We show that functionally intact core Alu elements are highly abundant and far outnumber all other active transposons in humans. A range of Alu lineages were found to harbor such copies, including all modern AluY subfamilies and most AluS subfamilies. We also identified two major determinants of Alu activity: (1) The primary sequence of a given Alu copy, and (2) the ability of the encoded RNA to interact with SRP9/14 to form RNA/protein (RNP) complexes. We conclude that Alu elements pose the largest transposon-based mutagenic threat to the human genome. On the basis of our data, we have begun to identify Alu copies that are likely to produce genetic variation and diseases in humans.
Figures


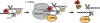
References
-
- Altman D.G., Machin D., Bryant T.N., Gardner M.J. Statistics with Confidence. BMJ books; Bristol, UK: 2000.
-
- Batzer M.A., Deininger P.L. Alu repeats and human genomic diversity. Nat. Rev. Genet. 2002;35:501–538. - PubMed
-
- Belancio V.P., Hedges D.J., Deininger P. Mammalian non-LTR retrotransposons: For better or worse, in sickness and in health. Genome Res. 2008;18:343–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
