Syntaxin 5 regulates the endoplasmic reticulum channel-release properties of polycystin-2
- PMID: 18836075
- PMCID: PMC2572927
- DOI: 10.1073/pnas.0805062105
Syntaxin 5 regulates the endoplasmic reticulum channel-release properties of polycystin-2
Abstract
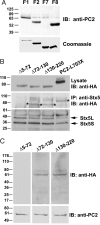
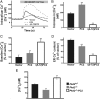
Polycystin-2 (PC2), the gene product of one of two genes mutated in dominant polycystic kidney disease, is a member of the transient receptor potential cation channel family and can function as intracellular calcium (Ca(2+)) release channel. We performed a yeast two-hybrid screen by using the NH(2) terminus of PC2 and identified syntaxin-5 (Stx5) as a putative interacting partner. Coimmunoprecipitation studies in cell lines and kidney tissues confirmed interaction of PC2 with Stx5 in vivo. In vitro binding assays showed that the interaction between Stx5 and PC2 is direct and defined the respective interaction domains as the t-SNARE region of Stx5 and amino acids 5 to 72 of PC2. Single channel studies showed that interaction with Stx5 specifically reduces PC2 channel activity. Epithelial cells overexpressing mutant PC2 that does not bind Stx5 had increased baseline cytosolic Ca(2+) levels, decreased endoplasmic reticulum (ER) Ca(2+) stores, and reduced Ca(2+) release from ER stores in response to vasopressin stimulation. Cells lacking PC2 altogether had reduced cytosolic Ca(2+) levels. Our data suggest that PC2 in the ER plays a role in cellular Ca(2+) homeostasis and that Stx5 functions to inactivate PC2 and prevent leaking of Ca(2+) from ER stores. Modulation of the PC2/Stx5 interaction may be a useful target for impacting dysregulated intracellular Ca(2+) signaling associated with polycystic kidney disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Adenylyl cyclase 5 links changes in calcium homeostasis to cAMP-dependent cyst growth in polycystic liver disease.J Hepatol. 2017 Mar;66(3):571-580. doi: 10.1016/j.jhep.2016.10.032. Epub 2016 Nov 5. J Hepatol. 2017. PMID: 27826057 Free PMC article.
-
Polycystin-2 is a novel cation channel implicated in defective intracellular Ca(2+) homeostasis in polycystic kidney disease.Biochem Biophys Res Commun. 2001 Mar 23;282(1):341-50. doi: 10.1006/bbrc.2001.4554. Biochem Biophys Res Commun. 2001. PMID: 11264013
-
Polycystin-2 (TRPP2): Ion channel properties and regulation.Gene. 2022 Jun 15;827:146313. doi: 10.1016/j.gene.2022.146313. Epub 2022 Mar 18. Gene. 2022. PMID: 35314260 Review.
-
A single amino acid residue constitutes the third dimerization domain essential for the assembly and function of the tetrameric polycystin-2 (TRPP2) channel.J Biol Chem. 2011 May 27;286(21):18994-9000. doi: 10.1074/jbc.M110.192286. Epub 2011 Apr 7. J Biol Chem. 2011. PMID: 21474446 Free PMC article.
-
Polycystin 2: A calcium channel, channel partner, and regulator of calcium homeostasis in ADPKD.Cell Signal. 2020 Feb;66:109490. doi: 10.1016/j.cellsig.2019.109490. Epub 2019 Dec 2. Cell Signal. 2020. PMID: 31805375 Free PMC article. Review.
Cited by
-
Polycystic liver diseases: congenital disorders of cholangiocyte signaling.Gastroenterology. 2011 Jun;140(7):1855-9, 1859.e1. doi: 10.1053/j.gastro.2011.04.030. Epub 2011 Apr 22. Gastroenterology. 2011. PMID: 21515270 Free PMC article. Review.
-
PKD2/polycystin-2 induces autophagy by forming a complex with BECN1.Autophagy. 2021 Jul;17(7):1714-1728. doi: 10.1080/15548627.2020.1782035. Epub 2020 Jun 30. Autophagy. 2021. PMID: 32543276 Free PMC article.
-
Polycystins and partners: proposed role in mechanosensitivity.J Physiol. 2014 Jun 15;592(12):2453-71. doi: 10.1113/jphysiol.2014.271346. Epub 2014 Mar 31. J Physiol. 2014. PMID: 24687583 Free PMC article. Review.
-
Valosin-containing protein-interacting membrane protein (VIMP) links the endoplasmic reticulum with microtubules in concert with cytoskeleton-linking membrane protein (CLIMP)-63.J Biol Chem. 2014 Aug 29;289(35):24304-13. doi: 10.1074/jbc.M114.571372. Epub 2014 Jul 9. J Biol Chem. 2014. PMID: 25008318 Free PMC article.
-
Posttranslational regulation of polycystin-2 protein expression as a novel mechanism of cholangiocyte reaction and repair from biliary damage.Hepatology. 2015 Dec;62(6):1828-39. doi: 10.1002/hep.28138. Epub 2015 Oct 10. Hepatology. 2015. Retraction in: Hepatology. 2022 Dec;76(6):1904. doi: 10.1002/hep.32595. PMID: 26313562 Free PMC article. Retracted.
References
-
- Somlo S, Torres VE, Caplan MJ. In: Seldin and Giebisch's The Kidney: Physiology & Pathophysiology. Alpern RJ, Hebert SC, editors. San Diego, CA: Academic; 2007. pp. 2283–2314.
-
- Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol. 2002;12:R378–R380. - PubMed
-
- Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13:2508–2516. - PubMed
-
- Cai Y, et al. Identification and characterization of polycystin-2, the PKD2 gene product. J Biol Chem. 1999;274:28557–28565. - PubMed
-
- Koulen P, et al. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol. 2002;4:191–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

