Petroleomics: chemistry of the underworld
- PMID: 18836082
- PMCID: PMC2587575
- DOI: 10.1073/pnas.0805069105
Petroleomics: chemistry of the underworld
Abstract
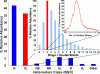
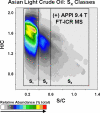
Each different molecular elemental composition-e.g., C(c)H(h)N(n)O(o)S(s)-has a different exact mass. With sufficiently high mass resolving power (m/Deltam(50%) approximately 400,000, in which m is molecular mass and Deltam(50%) is the mass spectral peak width at half-maximum peak height) and mass accuracy (<300 ppb) up to approximately 800 Da, now routinely available from high-field (>/=9.4 T) Fourier transform ion cyclotron resonance mass spectrometry, it is possible to resolve and identify uniquely and simultaneously each of the thousands of elemental compositions from the most complex natural organic mixtures, including petroleum crude oil. It is thus possible to separate and sort petroleum components according to their heteroatom class (N(n)O(o)S(s)), double bond equivalents (DBE = number of rings plus double bonds involving carbon, because each ring or double bond results in a loss of two hydrogen atoms), and carbon number. "Petroleomics" is the characterization of petroleum at the molecular level. From sufficiently complete characterization of the organic composition of petroleum and its products, it should be possible to correlate (and ultimately predict) their properties and behavior. Examples include molecular mass distribution, distillation profile, characterization of specific fractions without prior extraction or wet chemical separation from the original bulk material, biodegradation, maturity, water solubility (and oil:water emulsion behavior), deposits in oil wells and refineries, efficiency and specificity of catalytic hydroprocessing, "heavy ends" (asphaltenes) analysis, corrosion, etc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Smith DF, et al. Self-association of organic acids in petroleum and Canadian bitumen characterized by low- and high-resolution mass spectrometry. Energy Fuels. 2007;21:1309–1316.
-
- Marshall AG, Hendrickson CL, Jackson GS. Fourier transform ion cyclotron resonance mass spectrometry: A primer. Mass Spectrom Rev. 1998;17:1–35. - PubMed
-
- Hsu CS, Liang Z, Campana JE. Hydrocarbon characterization by ultrahigh resolution FTICRMS. Anal Chem. 1994;66:850–855.
-
- Guan S, Marshall AG, Scheppele SE. Resolution and chemical formula identification of aromatic hydrocarbons containing sulfur, nitrogen, and/or oxygen in crude oil distillates. Anal Chem. 1996;68:46–71. - PubMed
-
- Fenn JB, Mann M, Meng CK, Wong SF. Electrospray ionization–principles and practice. Electrospray Mass Spectrom Rev. 1990;9:37–70.
LinkOut - more resources
Full Text Sources
Other Literature Sources

