Purification, molecular cloning, and characterization of glutathione S-transferases (GSTs) from pigmented Vitis vinifera L. cell suspension cultures as putative anthocyanin transport proteins
- PMID: 18836188
- PMCID: PMC2561157
- DOI: 10.1093/jxb/ern217
Purification, molecular cloning, and characterization of glutathione S-transferases (GSTs) from pigmented Vitis vinifera L. cell suspension cultures as putative anthocyanin transport proteins
Abstract
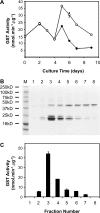
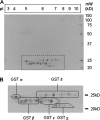
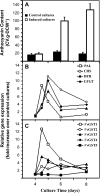
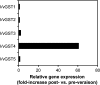
The ligandin activity of specific glutathione S-transferases (GSTs) is necessary for the transport of anthocyanins from the cytosol to the plant vacuole. Five GSTs were purified from Vitis vinifera L. cv. Gamay Fréaux cell suspension cultures by glutathione affinity chromatography. These proteins underwent Edman sequencing and mass spectrometry fingerprinting, with the resultant fragments aligned with predicted GSTs within public databases. The corresponding coding sequences were cloned, with heterologous expression in Escherichia coli used to confirm GST activity. Transcriptional profiling of these candidate GST genes and key anthocyanin biosynthetic pathway genes (PAL, CHS, DFR, and UFGT) in cell suspensions and grape berries against anthocyanin accumulation demonstrated strong positive correlation with two sequences, VvGST1 and VvGST4, respectively. The ability of VvGST1 and VvGST4 to transport anthocyanins was confirmed in the heterologous maize bronze-2 complementation model, providing further evidence for their function as anthocyanin transport proteins in grape cells. Furthermore, the differential induction of VvGST1 and VvGST4 in suspension cells and grape berries suggests functional differences between these two proteins. Further investigation of these candidate ligandins may identify a mechanism for manipulating anthocyanin accumulation in planta and in vitro suspension cells.
Figures



References
-
- Aharoni A, Keizer LC, van den Broeck HC, Blanco-Portales R, Munoz-Blanco J, Bois G, Smit P, de Vos RC, O'Connell AP. Novel insight into vascular, stress, and auxin-dependent and -independent gene expression programs in strawberry, a non-climacteric fruit. Plant Physiology. 2002;129:1019–1031. - PMC - PubMed
-
- Alonso JM, Stepanova AN, Leisse TJ, et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. - PubMed
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215:403–410. - PubMed
-
- Bézier A, Lambert B, Baillieul F. Study of defense-related gene expression in grapevine leaves and berries infected with Botrytis cinerea. European Journal of Plant Pathology. 2002;108:111–120.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

