Wnt-signaling and senescence: A tug of war in early neoplasia?
- PMID: 18836285
- PMCID: PMC2783518
- DOI: 10.4161/cbt.7.11.6943
Wnt-signaling and senescence: A tug of war in early neoplasia?
Abstract
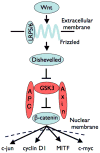
Studies of early neoplasia have revealed fundamental molecular pathways that drive tumorigenesis. Despite this progress, synthesis of principles of tumorigenesis that span tissue types has lagged. Such forays into the 'comparative anatomy' of cancer can stimulate new models and refine key questions. We envision commonality of pathways important in formation of two early benign neoplasms that are found in different tissues and which are not generally thought to be similar: dysplastic nevi of the skin and intestinal aberrant crypt foci. We propose that these neoplasms result from an ongoing 'tug of war' between the tumor suppression barrier posed by cellular senescence and the tumor-promoting activity of Wnt-signaling. Whether or not such neoplasms progress to malignancy or persist in a benign state for many years might be largely determined by the outcome of this tug of war and its modulation by other genetic and epigenetic alterations, such as inactivation of p16(INK4a).
Figures

References
-
- Nusse R, Brown A, Papkoff J, Scambler P, Shackleford G, McMahon A, Moon R, Varmus H. A new nomenclature for int-1 and related genes: the Wnt gene family. Cell. 1991;64:231. - PubMed
-
- Nusse R, van Ooyen A, Cox D, Fung YK, Varmus H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature. 1984;307:131–6. - PubMed
-
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
