High-speed, miniaturized fluorescence microscopy in freely moving mice
- PMID: 18836457
- PMCID: PMC2828344
- DOI: 10.1038/nmeth.1256
High-speed, miniaturized fluorescence microscopy in freely moving mice
Abstract
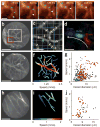
A central goal in biomedicine is to explain organismic behavior in terms of causal cellular processes. However, concurrent observation of mammalian behavior and underlying cellular dynamics has been a longstanding challenge. We describe a miniaturized (1.1 g mass) epifluorescence microscope for cellular-level brain imaging in freely moving mice, and its application to imaging microcirculation and neuronal Ca(2+) dynamics.
Figures


Comment in
-
New views into the brain of mice on the move.Nat Methods. 2008 Nov;5(11):925-6. doi: 10.1038/nmeth1108-925. Nat Methods. 2008. PMID: 18974733 No abstract available.
References
-
- Ferezou I, Bolea S, Petersen CC. Neuron. 2006;50:617–629. - PubMed
-
- Yamaguchi S, et al. Nature. 2001;409:684. - PubMed
-
- Adelsberger H, Garaschuk O, Konnerth A. Nat Neurosci. 2005;8:988–990. - PubMed
-
- Murayama M, Perez-Garci E, Luscher HR, Larkum ME. J Neurophysiol. 2007;98:1791–1805. - PubMed
-
- Poe GR, Kristensen MP, Rector DM, Harper RM. Neuroscience. 1996;72:39–48. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

