Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis
- PMID: 18836458
- PMCID: PMC2751601
- DOI: 10.1038/nm.1874
Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis
Abstract
Pauci-immune focal necrotizing glomerulonephritis (FNGN) is a severe inflammatory disease associated with autoantibodies to neutrophil cytoplasmic antigens (ANCA). Here we characterize autoantibodies to lysosomal membrane protein-2 (LAMP-2) and show that they are a new ANCA subtype present in almost all individuals with FNGN. Consequently, its prevalence is nearly twice that of the classical ANCAs that recognize myeloperoxidase or proteinase-3. Furthermore, antibodies to LAMP-2 cause pauci-immune FNGN when injected into rats, and a monoclonal antibody to human LAMP-2 (H4B4) induces apoptosis of human microvascular endothelium in vitro. The autoantibodies in individuals with pauci-immune FNGN commonly recognize a human LAMP-2 epitope (designated P(41-49)) with 100% homology to the bacterial adhesin FimH, with which they cross-react. Rats immunized with FimH develop pauci-immune FNGN and also develop antibodies to rat and human LAMP-2. Finally, we show that infections with fimbriated pathogens are common before the onset of FNGN. Thus, FimH-triggered autoimmunity to LAMP-2 provides a previously undescribed clinically relevant molecular mechanism for the development of pauci-immune FNGN.
Figures

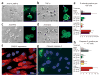


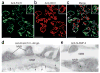
Comment in
-
Autoantibodies vex the vasculature.Nat Med. 2008 Oct;14(10):1018-9. doi: 10.1038/nm1008-1018. Nat Med. 2008. PMID: 18841136 No abstract available.
-
Vasculitis syndromes: LAMP-2 illuminates pathogenesis of ANCA glomerulonephritis.Nat Rev Nephrol. 2009 May;5(5):247-9. doi: 10.1038/nrneph.2009.51. Nat Rev Nephrol. 2009. PMID: 19384321
-
LAMPs and NETs in the pathogenesis of ANCA vasculitis.J Am Soc Nephrol. 2009 Aug;20(8):1654-6. doi: 10.1681/ASN.2009060616. Epub 2009 Jul 16. J Am Soc Nephrol. 2009. PMID: 19608698 No abstract available.
References
-
- Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337:1512–1523. - PubMed
-
- Morgan MD, Harper L, Williams J, Savage C. Anti-neutrophil cytoplasm associated glomerulonephritis. J Am Soc Nephrol. 2006;17:1224–1234. - PubMed
-
- van der Woude FJ, et al. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener’s granulomatosis. Lancet. 1985;1:425–429. - PubMed
-
- Jennette JC, Xiao H, Falk RJ. Pathogenesis of vascular inflammation by anti-neutrophil cytoplasmic antibodies. J Am Soc Nephrol. 2006;17:1235–1242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

