Chemical control of protein stability and function in living mice
- PMID: 18836461
- PMCID: PMC2605277
- DOI: 10.1038/nm.1754
Chemical control of protein stability and function in living mice
Abstract
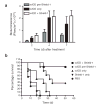
Conditional control of protein function in vivo offers great potential for deconvoluting the roles of individual proteins in complicated systems. We recently developed a method in which a small protein domain, termed a destabilizing domain, confers instability to fusion protein partners in cultured cells. Instability is reversed when a cell-permeable small molecule binds this domain. Here we describe the use of this system to regulate protein function in living mammals. We show regulation of secreted proteins and their biological activity with conditional secretion of an immunomodulatory cytokine, resulting in tumor burden reduction in mouse models. Additionally, we use this approach to control the function of a specific protein after systemic delivery of the gene that encodes it to a tumor, suggesting uses for enhancing the specificity and efficacy of targeted gene-based therapies. This method represents a new strategy to regulate protein function in living organisms with a high level of control.
Figures

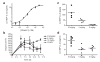

References
-
- Bockamp E, et al. Of mice and models: improved animal models for biomedical research. Physiol Genomics. 2002;11:115–132. - PubMed
-
- Bradley A, Evans M, Kaufman MH, Robertson E. Formation of germ-line chimeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–256. - PubMed
-
- Thomas KR, Capecchi MR. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987;51:503–512. - PubMed
-
- Doetschman T, et al. Targetted correction of a mutant HPRT gene in mouse embryonic stem cells. Nature. 1987;330:576–578. - PubMed
-
- Yamada G, et al. Targeted mutation of the murine goosecoid gene results in craniofacial defects and neonatal death. Development. 1995;121:2917–2922. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

