TRIM family proteins and their emerging roles in innate immunity
- PMID: 18836477
- PMCID: PMC3433745
- DOI: 10.1038/nri2413
TRIM family proteins and their emerging roles in innate immunity
Abstract
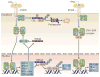
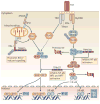
The superfamily of tripartite motif-containing (TRIM) proteins is conserved throughout the metazoan kingdom and has expanded rapidly during vertebrate evolution; there are now more than 60 TRIM proteins known in humans and mice. Many TRIM proteins are induced by type I and type II interferons, which are crucial for many aspects of resistance to pathogens, and several are known to be required for the restriction of infection by lentiviruses. In this Review, we describe recent data that reveal broader antiviral and antimicrobial activities of TRIM proteins and discuss their involvement in the regulation of pathogen-recognition and transcriptional pathways in host defence.
Figures

References
-
- Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. - PubMed
-
- O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nature Rev Immunol. 2007;7:353–364. - PubMed
-
- Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-γ. Annu Rev Immunol. 1997;15:749–795. - PubMed
-
- Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. - PubMed
-
- Beutler B, et al. Genetic analysis of resistance to viral infection. Nature Rev Immunol. 2007;7:753–766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

