Impact of efflux transporters and of seizures on the pharmacokinetics of oxcarbazepine metabolite in the rat brain
- PMID: 18836479
- PMCID: PMC2597256
- DOI: 10.1038/bjp.2008.366
Impact of efflux transporters and of seizures on the pharmacokinetics of oxcarbazepine metabolite in the rat brain
Abstract
Background and purpose: Accurate prediction of biophase pharmacokinetics (PK) is essential to optimize pharmacotherapy in epilepsy. Here, we characterized the PK of the active metabolite of oxcarbazepine, 10,11-dihydro-10-hydroxy-carbamazepine (MHD) in plasma and in the hippocampus. Simultaneously, the impact of acute seizures and efflux transport mechanisms on brain distribution was quantified.
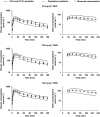
Experimental approach: Rats received subtherapeutic and anticonvulsant doses of MHD in non-epileptic conditions and during focal pilocarpine-induced limbic seizures. To evaluate the effect of efflux transport blockade, a separate group received subtherapeutic doses combined with intrahippocampal perfusion of verapamil. Free plasma and extracellular hippocampal MHD concentrations were determined using microdialysis and liquid chromatography techniques. An integrated PK model describing simultaneously the PK of MHD in plasma and brain was developed using nonlinear mixed effects modelling. A bootstrap procedure and a visual predictive check were performed to assess model performance.
Key results: A compartmental model with combined zero- and first-order absorption, including lag time and biophase distribution best described the PK of MHD. A distributional process appeared to underlie the increased brain MHD concentrations observed following seizure activity and efflux transport inhibition, as reflected by changes in the volume of distribution of the biophase compartment. In contrast, no changes were observed in plasma PK.
Conclusions and implications: Simultaneous PK modelling of plasma and brain concentrations has not been used previously in the evaluation of antiepileptic drugs (AEDs). Characterisation of biophase PK is critical to assess the impact of efflux transport mechanisms and acute seizures on brain disposition and, consequently, on AED effects.
Figures






Similar articles
-
Hippocampal dopamine and serotonin elevations as pharmacodynamic markers for the anticonvulsant efficacy of oxcarbazepine and 10,11-dihydro-10-hydroxycarbamazepine.Neurosci Lett. 2005 Dec 16;390(1):48-53. doi: 10.1016/j.neulet.2005.07.049. Neurosci Lett. 2005. PMID: 16139430
-
Differential hippocampal pharmacokinetics of phenobarbital and carbamazepine in repetitive seizures induced by 3-mercaptopropionic acid.Neurosci Lett. 2009 Mar 27;453(1):54-7. doi: 10.1016/j.neulet.2009.01.079. Epub 2009 Feb 4. Neurosci Lett. 2009. PMID: 19429015
-
Quantitative in vivo microdialysis study on the influence of multidrug transporters on the blood-brain barrier passage of oxcarbazepine: concomitant use of hippocampal monoamines as pharmacodynamic markers for the anticonvulsant activity.J Pharmacol Exp Ther. 2005 Aug;314(2):725-31. doi: 10.1124/jpet.105.085514. Epub 2005 Apr 28. J Pharmacol Exp Ther. 2005. PMID: 15860570
-
Prediction of antiepileptic drug efficacy: the use of intracerebral microdialysis to monitor biophase concentrations.Expert Opin Drug Metab Toxicol. 2009 Oct;5(10):1267-77. doi: 10.1517/17425250903146903. Expert Opin Drug Metab Toxicol. 2009. PMID: 19611404 Review.
-
Clinical pharmacokinetics of oxcarbazepine.Clin Pharmacokinet. 2003;42(12):1023-42. doi: 10.2165/00003088-200342120-00002. Clin Pharmacokinet. 2003. PMID: 12959634 Review.
Cited by
-
Implications of BCRP modulation on PTZ-induced seizures in mice: Role of ko143 and metformin as adjuvants to lamotrigine.Naunyn Schmiedebergs Arch Pharmacol. 2023 Oct;396(10):2627-2636. doi: 10.1007/s00210-023-02485-7. Epub 2023 Apr 17. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 37067582 Free PMC article.
-
The mastermind approach to CNS drug therapy: translational prediction of human brain distribution, target site kinetics, and therapeutic effects.Fluids Barriers CNS. 2013 Feb 22;10(1):12. doi: 10.1186/2045-8118-10-12. Fluids Barriers CNS. 2013. PMID: 23432852 Free PMC article.
-
Central IGF-I Receptors in the Brain are Instrumental to Neuroprotection by Systemically Injected IGF-I in a Rat Model for Ischemic Stroke.CNS Neurosci Ther. 2016 Jul;22(7):611-6. doi: 10.1111/cns.12550. Epub 2016 Apr 15. CNS Neurosci Ther. 2016. PMID: 27080541 Free PMC article.
References
-
- Beal SL, Sheiner LB. NONMEM Users Guide. University of California: San Francisco, CA; 1999.
-
- Benveniste H, Hüttemeier P. Microdialysis—theory and application. Prog Neurobiol. 1990;35:195–215. - PubMed
-
- Clapp-Lilly K, Roberts R, Duffy L, Irons K, Hu Y, Drew K. An ultrastructural analysis of tissue surrounding a microdialysis probe. J Neurosci Methods. 1999;90:129–142. - PubMed
-
- Cleton A, de Greef H, Edelbroek P, Voskuyl R, Danhof M. Application of a combined ‘effect compartment/indirect response model' to the central nervous system effects of tiagabine in the rat. J Pharmacokinet Biopharm. 1999;27:301–323. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

