Her-2 DNA versus cell vaccine: immunogenicity and anti-tumor activity
- PMID: 18836716
- PMCID: PMC3827718
- DOI: 10.1007/s00262-008-0599-x
Her-2 DNA versus cell vaccine: immunogenicity and anti-tumor activity
Abstract
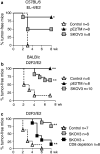
Direct comparison and ranking of vaccine formulations in pre-clinical studies will expedite the identification of cancer vaccines for clinical trials. Two human ErbB-2 (Her-2) vaccines, naked DNA and whole cell vaccine, were tested side-by-side in wild type and Her-2 transgenic mice. Both vaccines can induce humoral and cellular immunity to the entire repertoire of Her-2 epitopes. Mice were electro-vaccinated i.m. with a mixture of pGM-CSF and pE2TM, the latter encodes Her-2 extracellular and transmembrane domains. Alternatively, mice were injected i.p. with human ovarian cancer SKOV3 cells that have amplified Her-2. In wild type mice, comparable levels of Her-2 antibodies (Ab) were induced by these two vaccines. However, T cell immunity and protection against Her-2(+) tumors were superior in DNA vaccinated mice. In BALB Her-2 transgenic (Tg) mice, which were tolerant to Her-2, DNA and cell vaccines were administered after regulatory T cells (Treg) were removed by anti-CD25 mAb. Again, comparable levels of Her-2 Ab were induced, but DNA vaccines rendered greater anti-tumor activity. In B6xDR3 Her-2 Tg mice that expressed the autoimmune prone HLA-DR3 allele, higher levels of Her-2 Ab were induced by SKOV3 cell than by Her-2 DNA. But anti-tumor activity was still more profound in DNA vaccinated mice. Therefore, Her-2 DNA vaccine induced greater anti-tumor immunity than cell vaccine, whether mice were tolerant to Her-2 or susceptible to autoimmunity. Through such side-by-side comparisons in appropriate pre-clinical test systems, the more effective vaccine formulations will emerge as candidates for clinical trials.
Figures




Similar articles
-
Activity of DNA vaccines encoding self or heterologous Her-2/neu in Her-2 or neu transgenic mice.Cell Immunol. 2006 Apr;240(2):96-106. doi: 10.1016/j.cellimm.2006.07.002. Epub 2006 Aug 22. Cell Immunol. 2006. PMID: 16930573
-
Control of Her-2 tumor immunity and thyroid autoimmunity by MHC and regulatory T cells.Cancer Res. 2007 Jul 15;67(14):7020-7. doi: 10.1158/0008-5472.CAN-06-4755. Cancer Res. 2007. PMID: 17638915
-
Dual antigen target-based immunotherapy for prostate cancer eliminates the growth of established tumors in mice.Immunotherapy. 2011 Jun;3(6):735-46. doi: 10.2217/imt.11.59. Immunotherapy. 2011. PMID: 21668311
-
HER-2/neu as a target for cancer vaccines.Immunotherapy. 2010 Mar;2(2):213-26. doi: 10.2217/imt.09.89. Immunotherapy. 2010. PMID: 20635929 Review.
-
The "A, B and C" of Her-2 DNA vaccine development.Cancer Immunol Immunother. 2008 Nov;57(11):1711-7. doi: 10.1007/s00262-008-0464-y. Epub 2008 Feb 14. Cancer Immunol Immunother. 2008. PMID: 18273615 Free PMC article. Review.
Cited by
-
Breast cancer vaccines delivered by dendritic cell-targeted lentivectors induce potent antitumor immune responses and protect mice from mammary tumor growth.Vaccine. 2017 Oct 13;35(43):5842-5849. doi: 10.1016/j.vaccine.2017.09.017. Epub 2017 Sep 12. Vaccine. 2017. PMID: 28916248 Free PMC article.
-
Evolution of animal models in cancer vaccine development.Vaccine. 2015 Dec 16;33(51):7401-7407. doi: 10.1016/j.vaccine.2015.07.075. Epub 2015 Aug 1. Vaccine. 2015. PMID: 26241945 Free PMC article. Review.
-
Antigen-Clustered Nanovaccine Achieves Long-Term Tumor Remission by Promoting B/CD 4 T Cell Crosstalk.ACS Nano. 2024 Apr 2;18(13):9584-9604. doi: 10.1021/acsnano.3c13038. Epub 2024 Mar 21. ACS Nano. 2024. PMID: 38513119 Free PMC article.
-
Comparison of prophylactic and therapeutic immunisation with an ErbB-2 (HER2) fusion protein and immunoglobulin V-gene repertoire analysis in a transgenic mouse model of spontaneous breast cancer.Vaccine. 2014 Feb 12;32(8):1012-8. doi: 10.1016/j.vaccine.2013.10.077. Epub 2013 Nov 11. Vaccine. 2014. PMID: 24231440 Free PMC article.
-
Immunotherapeutic intervention with oncolytic adenovirus in mouse mammary tumors.Oncoimmunology. 2015 Feb 3;4(1):e984523. doi: 10.4161/2162402X.2014.984523. eCollection 2015 Jan. Oncoimmunology. 2015. PMID: 25949865 Free PMC article.
References
-
- de Vries RR, Huizinga TW, Toes RE. Redefining the HLA and RA association: to be or not to be anti-CCP positive. J Autoimmun. 2005;25 Suppl:21–25. - PubMed
-
- Disis ML, Grabstein KH, Sleath PR, et al. Generation of immunity to the HER-2/neu oncogenic protein in patients with breast and ovarian cancer using a peptide-based vaccine. Clin Cancer Res. 1999;5:1289–1297. - PubMed
-
- Dols A, Smith JW, Meijer SL, et al. Vaccination of women with metastatic breast cancer, using a costimulatory gene (CD80)-modified, HLA-A2-matched, allogeneic, breast cancer cell line: clinical and immunological results. Hum Gene Ther. 2003;14:1117–1123. doi: 10.1089/104303403322124828. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

