Highly sensitive and selective colorimetric sensors for uranyl (UO2(2+)): development and comparison of labeled and label-free DNAzyme-gold nanoparticle systems
- PMID: 18837498
- PMCID: PMC2667950
- DOI: 10.1021/ja803607z
Highly sensitive and selective colorimetric sensors for uranyl (UO2(2+)): development and comparison of labeled and label-free DNAzyme-gold nanoparticle systems
Abstract
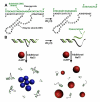
Colorimetric uranium sensors based on uranyl (UO2(2+)) specific DNAzyme and gold nanoparticles (AuNP) have been developed and demonstrated using both labeled and label-free methods. In the labeled method, a uranyl-specific DNAzyme was attached to AuNP, forming purple aggregates. The presence of uranyl induced disassembly of the DNAzyme functionalized AuNP aggregates, resulting in red individual AuNPs. Once assembled, such a "turn-on" sensor is highly stable, works in a single step at room temperature, and has a detection limit of 50 nM after 30 min of reaction time. The label-free method, on the other hand, utilizes the different adsorption properties of single-stranded and double-stranded DNA on AuNPs, which affects the stability of AuNPs in the presence of NaCl. The presence of uranyl resulted in cleavage of substrate by DNAzyme, releasing a single stranded DNA that can be adsorbed on AuNPs and protect them from aggregation. Taking advantage of this phenomenon, a "turn-off" sensor was developed, which is easy to control through reaction quenching and has 1 nM detection limit after 6 min of reaction at room temperature. Both sensors have excellent selectivity over other metal ions and have detection limits below the maximum contamination level of 130 nM for UO2(2+) in drinking water defined by the U.S. Environmental Protection Agency (EPA). This study represents the first direct systematic comparison of these two types of sensor methods using the same DNAzyme and AuNPs, making it possible to reveal advantages, disadvantages, versatility, limitations, and potential applications of each method. The results obtained not only allow practical sensing application for uranyl but also serve as a guide for choosing different methods for designing colorimetric sensors for other targets.
Figures






Similar articles
-
Resonance light scattering determination of uranyl based on labeled DNAzyme-gold nanoparticle system.Spectrochim Acta A Mol Biomol Spectrosc. 2013 Jun;110:419-24. doi: 10.1016/j.saa.2013.03.036. Epub 2013 Mar 14. Spectrochim Acta A Mol Biomol Spectrosc. 2013. PMID: 23583878
-
A DNAzyme-gold nanoparticle probe for uranyl ion in living cells.J Am Chem Soc. 2013 Apr 10;135(14):5254-7. doi: 10.1021/ja400150v. Epub 2013 Mar 26. J Am Chem Soc. 2013. PMID: 23531046 Free PMC article.
-
Magnetic beads-based DNAzyme recognition and AuNPs-based enzymatic catalysis amplification for visual detection of trace uranyl ion in aqueous environment.Biosens Bioelectron. 2016 Apr 15;78:73-79. doi: 10.1016/j.bios.2015.11.024. Epub 2015 Nov 12. Biosens Bioelectron. 2016. PMID: 26594889
-
DNAzyme-functionalized gold nanoparticles for biosensing.Adv Biochem Eng Biotechnol. 2014;140:93-120. doi: 10.1007/10_2013_242. Adv Biochem Eng Biotechnol. 2014. PMID: 24026635 Review.
-
Colorimetric biosensors based on DNAzyme-assembled gold nanoparticles.J Fluoresc. 2004 Jul;14(4):343-54. doi: 10.1023/b:jofl.0000031816.06134.d3. J Fluoresc. 2004. PMID: 15617377 Review.
Cited by
-
SERS detection of uranyl based on MOF-coated gold nanooctahedron hybrid.Anal Sci. 2024 Dec;40(12):2111-2116. doi: 10.1007/s44211-024-00646-z. Epub 2024 Aug 24. Anal Sci. 2024. PMID: 39180664
-
Uranyl Binding to Proteins and Structural-Functional Impacts.Biomolecules. 2020 Mar 16;10(3):457. doi: 10.3390/biom10030457. Biomolecules. 2020. PMID: 32187982 Free PMC article. Review.
-
A protein engineered to bind uranyl selectively and with femtomolar affinity.Nat Chem. 2014 Mar;6(3):236-41. doi: 10.1038/nchem.1856. Epub 2014 Jan 26. Nat Chem. 2014. PMID: 24557139
-
DNA-Functionalized Nanoparticles for Targeted Biosensing and Biological Applications.ACS Omega. 2020 Nov 28;5(48):30767-30774. doi: 10.1021/acsomega.0c03656. eCollection 2020 Dec 8. ACS Omega. 2020. PMID: 33324786 Free PMC article. Review.
-
Strategy for increasing drug solubility and efficacy through covalent attachment to polyvalent DNA-nanoparticle conjugates.ACS Nano. 2011 Sep 27;5(9):6962-70. doi: 10.1021/nn201446c. Epub 2011 Aug 12. ACS Nano. 2011. PMID: 21812457 Free PMC article.
References
-
- Konstantin B. Gongalsky. Environ. Monit. Assess. 2003;89:197–219. - PubMed
-
- Craft E, Abu-Qare A, Flaherty M, Garofolo M, Rincavage H, Abou-Donia M. J. Toxicol. Environ. Health B Crit. Rev. 2004;7:297–317. - PubMed
-
- Zhou P, Gu B. Environ. Sci. Technol. 2005;39:4435–4440. - PubMed
-
- Abbasi SA. Int. J. Environ. Anal. Chem. 1989;36:163–172. - PubMed
-
- Brina R, Miller AG. Anal. Chem. 1992;64:1413–1418.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

