Dscam-mediated cell recognition regulates neural circuit formation
- PMID: 18837673
- PMCID: PMC2711549
- DOI: 10.1146/annurev.cellbio.24.110707.175250
Dscam-mediated cell recognition regulates neural circuit formation
Abstract
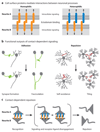
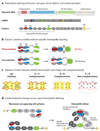
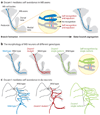
The Dscam family of immunoglobulin cell surface proteins mediates recognition events between neurons that play an essential role in the establishment of neural circuits. The Drosophila Dscam1 locus encodes tens of thousands of cell surface proteins via alternative splicing. These isoforms exhibit exquisite isoform-specific binding in vitro that mediates homophilic repulsion in vivo. These properties provide the molecular basis for self-avoidance, an essential developmental mechanism that allows axonal and dendritic processes to uniformly cover their synaptic fields. In a mechanistically similar fashion, homophilic repulsion mediated by Drosophila Dscam2 prevents processes from the same class of cells from occupying overlapping synaptic fields through a process called tiling. Genetic studies in the mouse visual system support the view that vertebrate DSCAM also promotes both self-avoidance and tiling. By contrast, DSCAM and DSCAM-L promote layer-specific targeting in the chick visual system, presumably through promoting homophilic adhesion. The fly and mouse studies underscore the importance of homophilic repulsion in regulating neural circuit assembly, whereas the chick studies suggest that DSCAM proteins may mediate a variety of different recognition events during wiring in a context-dependent fashion.
Figures

References
-
- Agarwala KL, Ganesh S, Tsutsumi Y, Suzuki T, Amano K, Yamakawa K. Cloning and functional characterization of DSCAML1, a novel DSCAM-like cell adhesion molecule that mediates homophilic intercellular adhesion. Biochem. Biophys. Res. Commun. 2001;285:760–772. - PubMed
-
- Baker MW, Macagno ER. The role of a LAR-like receptor tyrosine phosphatase in growth cone collapse and mutual-avoidance by sibling processes. J. Neurobiol. 2000;4:194–203. - PubMed
-
-
Chen BE, Kondo M, Garnier A, Watson FL, Puettmann-Holgado R, et al. The molecular diversity of Dscam is functionally required for neuronal wiring specificity in Drosophila. Cell. 2006;125:607–620. Demonstrated branching defects in Dscam1 mutants lacking specific exon 4 variants, suggesting a role for specific isoforms in patterning connections.
-
-
- Cheng HJ, Flanagan JG. Identification and cloning of ELF-1, a developmentally expressed ligand for the Mek4 and Sek receptor tyrosine kinases. Cell. 1994;79:157–168. - PubMed
-
- Drescher U, Kremoser C, Handwerker C, Loschinger J, Noda M, Bonhoeffer F. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995;82:359–370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

