The structural immunology of antibody protection against West Nile virus
- PMID: 18837784
- PMCID: PMC2646609
- DOI: 10.1111/j.1600-065X.2008.00676.x
The structural immunology of antibody protection against West Nile virus
Abstract
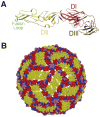
Recent investigations of the interaction between the West Nile virus (WNV) envelope protein (E) and monoclonal antibodies (mAbs) have elucidated fundamental insights into the molecular mechanisms of neutralization. Structural studies have defined an epitope on the lateral ridge of domain III (DIII-lr) of the WNV E protein that is recognized by antibodies with the strongest neutralizing activity in vitro and in vivo. Antibodies that bind this epitope are highly potent because they efficiently block at a post-entry step of viral infection with relatively low virion occupancy requirements. In this review, we discuss the structural, molecular, and immunologic basis for antibody-mediated protection against WNV, and its implications for novel therapeutic or vaccine strategies.
Figures



References
-
- Sejvar JJ, et al. Neurologic manifestations and outcome of West Nile virus infection. JAMA. 2003;290:511–515. - PubMed
-
- Lanciotti RS, et al. Complete genome sequences and phylogenetic analysis of West Nile virus strains isolated from the United States, Europe, and the Middle East. Virology. 2002;298:96–105. - PubMed
-
- Beasley DW, Li L, Suderman MT, Barrett AD. Mouse neuroinvasive phenotype of West Nile virus strains varies depending upon virus genotype. Virology. 2002;296:17–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

