Non invasive in vivo investigation of hepatobiliary structure and function in STII medaka (Oryzias latipes): methodology and applications
- PMID: 18838008
- PMCID: PMC2586619
- DOI: 10.1186/1476-5926-7-7
Non invasive in vivo investigation of hepatobiliary structure and function in STII medaka (Oryzias latipes): methodology and applications
Abstract
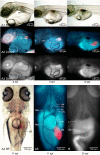
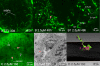
Background: A novel transparent stock of medaka (Oryzias latipes; STII), recessive for all pigments found in chromatophores, permits transcutaneous imaging of internal organs and tissues in living individuals. Findings presented describe the development of methodologies for non invasive in vivo investigation in STII medaka, and the successful application of these methodologies to in vivo study of hepatobiliary structure, function, and xenobiotic response, in both 2 and 3 dimensions.
Results: Using brightfield, and widefield and confocal fluorescence microscopy, coupled with the in vivo application of fluorescent probes, structural and functional features of the hepatobiliary system, and xenobiotic induced toxicity, were imaged at the cellular level, with high resolution (< 1 microm), in living individuals. The findings presented demonstrate; (1) phenotypic response to xenobiotic exposure can be investigated/imaged in vivo with high resolution (< 1 microm), (2) hepatobiliary transport of solutes from blood to bile can be qualitatively and quantitatively studied/imaged in vivo, (3) hepatobiliary architecture in this lower vertebrate liver can be studied in 3 dimensions, and (4) non invasive in vivo imaging/description of hepatobiliary development in this model can be investigated.
Conclusion: The non-invasive in vivo methodologies described are a unique means by which to investigate biological structure, function and xenobiotic response with high resolution in STII medaka. In vivo methodologies also provide the future opportunity to integrate molecular mechanisms (e.g., genomic, proteomic) of disease and toxicity with phenotypic changes at the cellular and system levels of biological organization. While our focus has been the hepatobiliary system, other organ systems are equally amenable to in vivo study, and we consider the potential for discovery, within the context of in vivo investigation in STII medaka, as significant.
Figures







Similar articles
-
Non invasive high resolution in vivo imaging of alpha-naphthylisothiocyanate (ANIT) induced hepatobiliary toxicity in STII medaka.Aquat Toxicol. 2008 Jan 20;86(1):20-37. doi: 10.1016/j.aquatox.2007.09.014. Epub 2007 Oct 6. Aquat Toxicol. 2008. PMID: 18022256 Free PMC article.
-
An in vivo look at vertebrate liver architecture: three-dimensional reconstructions from medaka (Oryzias latipes).Anat Rec (Hoboken). 2007 Jul;290(7):770-82. doi: 10.1002/ar.20524. Anat Rec (Hoboken). 2007. PMID: 17516461
-
Protective response of the Ah receptor to ANIT-induced biliary epithelial cell toxicity in see-through medaka.Toxicol Sci. 2008 Apr;102(2):262-77. doi: 10.1093/toxsci/kfm308. Epub 2008 Jan 10. Toxicol Sci. 2008. PMID: 18187559
-
Diagnostic criteria for degenerative, inflammatory, proliferative nonneoplastic and neoplastic liver lesions in medaka (Oryzias latipes): consensus of a National Toxicology Program Pathology Working Group.Toxicol Pathol. 1997 Mar-Apr;25(2):202-10. doi: 10.1177/019262339702500210. Toxicol Pathol. 1997. PMID: 9125779 Review.
-
The National BioResource Project Medaka (NBRP Medaka): an integrated bioresource for biological and biomedical sciences.Exp Anim. 2010;59(1):13-23. doi: 10.1538/expanim.59.13. Exp Anim. 2010. PMID: 20224166 Review.
Cited by
-
Anchoring hepatic gene expression with development of fibrosis and neoplasia in a toxicant-induced fish model of liver injury.Toxicol Pathol. 2013 Jul;41(5):744-60. doi: 10.1177/0192623312464308. Epub 2012 Nov 28. Toxicol Pathol. 2013. PMID: 23197195 Free PMC article.
-
Non invasive high resolution in vivo imaging of alpha-naphthylisothiocyanate (ANIT) induced hepatobiliary toxicity in STII medaka.Aquat Toxicol. 2008 Jan 20;86(1):20-37. doi: 10.1016/j.aquatox.2007.09.014. Epub 2007 Oct 6. Aquat Toxicol. 2008. PMID: 18022256 Free PMC article.
References
-
- Alpini G, Glaser S, Alvaro D, Ueno Y, Marzioni M, Francis H, Baiocchi L, Stati T, Barbaro B, Phinizy JL, Mauldin J, Lesage G. Bile acid depletion and repletion regulate cholangiocyte growth and secretion by a phosphatidylinositol 3-kinase-dependent pathway in rats. Gastroenterology. 2002;123:1226–1237. doi: 10.1053/gast.2002.36055. - DOI - PubMed
-
- Boyer JL. Bile duct epithelium: frontiers in transport physiology. Am J Physiol. 1996;270:G1–5. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
