A re-assessment of minocycline as a neuroprotective agent in a rat spinal cord contusion model
- PMID: 18838063
- PMCID: PMC2643041
- DOI: 10.1016/j.brainres.2008.09.047
A re-assessment of minocycline as a neuroprotective agent in a rat spinal cord contusion model
Abstract
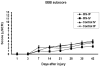
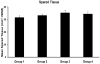
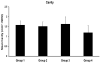
This study was initiated due to an NIH "Facilities of Research--Spinal Cord Injury" contract to support independent replication of published studies that could be considered for a clinical trial in time. Minocycline has been shown to have neuroprotective effects in models of central nervous system injury, including in a contusive spinal cord injury (SCI) model at the thoracic level. Beneficial effects of minocycline treatment included a significant improvement in locomotor behavior and reduced histopathological changes [Lee, S.M., Yune, T.Y., Kim, S.J., Park, D.O.W., Lee, Y.K., Kim, Y.C., Oh, Y.J., Markelonis, G.J., Oh, T.H., 2003. Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J Neurotrauma. 20, 1017-1027.] To verify these important observations, we repeated this study in our laboratory. The NYU (MASCIS) Impactor was used to produce a moderate cord lesion at the vertebral level T9-T10 (height 12.5 mm, weight 10 g), (n=45), followed by administration of minocycline, 90 mg/kg (group 1: minocycline IP, n=15; group 2: minocycline IV, n=15; group 3: vehicle IP, n=8; group 4: vehicle IV, n=7) immediately after surgery and followed by two more doses of 45 mg/kg/IP at 12 h and 24 h. Open field locomotion (BBB) and subscores were examined up to 6 weeks after SCI and cords were processed for quantitative histopathological analysis. Administration of minocycline after SCI did not lead to significant behavioral or histopathological improvement. Although positive effects with minocycline have been reported in several animal models of injury with different drug administration schemes, the use of minocycline following contusive SCI requires further investigation before clinical trials are implemented.
Figures


References
-
- Basso DM, Beattie MS, Bresnahan JC, Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ, Nockels R, Perot PL, Salzman SK, Young W. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma. 1996;13:343–359. - PubMed
-
- Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, Friedlander RM. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med. 2000;6:797–801. - PubMed
-
- Diguet E, Fernagut PO, Wei X, Du Y, Rouland R, Gross C, Bezard E, Tison F. Deleterious effects of minocycline in animal models of Parkinson's disease and Huntington's disease. Eur J Neurosci. 2004;19:3266–3276. - PubMed
-
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. - PubMed
-
- Fagan SC, Edwards DJ, Borlongan CV, Xu L, Arora A, Feuerstein G, Hess DC. Optimal delivery of minocycline to the brain: implication for human studies of acute neuroprotection. Exp Neurol. 2004;186:248–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

