Kisspeptin and seasonality in sheep
- PMID: 18838092
- PMCID: PMC3712824
- DOI: 10.1016/j.peptides.2008.08.022
Kisspeptin and seasonality in sheep
Abstract
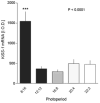
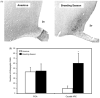
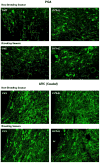
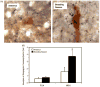
Sheep are seasonal breeders, experiencing a period of reproductive quiescence during spring and early summer. During the non-breeding period, kisspeptin expression in the arcuate nucleus is markedly reduced. This strongly suggests that the mechanisms that control seasonal changes in reproductive function involve kisspeptin neurons. Kisspeptin cells appear to regulate GnRH neurons and transmit sex-steroid feedback to the reproductive axis. Since the non-breeding season is characterized by increased negative feedback of estrogen on GnRH secretion, the kisspeptin neurons seem to be fundamentally involved in the determination of breeding state. The reduction in kisspeptin neuronal function during the non-breeding season can be corrected by infusion of kisspeptin, which causes ovulation in seasonally acyclic females.
Figures


References
-
- Adams VL, Goodman RL, Salm AK, Coolen LM, Karsch FJ, Lehman MN. Morphological plasticity in the neural circuitry responsible for seasonal breeding in the ewe. Endocrinology. 2006;147:4843–4851. - PubMed
-
- Bentley GE, Perfito N, Ukena K, Tsutsui K, Wingfield JC. Gonadotropin-inhibitory peptide in song sparrows (Melospiza melodia) in different reproductive conditions, and in house sparrows (Passer domesticus) relative to chicken-gonadotropin-releasing hormone. J Neuroendocrinol. 2003;15:794–802. - PubMed
-
- Caraty A, Fabre-Nys C, Delaleu B, Locatelli A, Bruneau G, Karsch FJ, Herbison A. Evidence that the mediobasal hypothalamus is the primary site of action of estradiol in inducing the preovulatory gonadotropin releasing hormone surge in the ewe. Endocrinology. 1998;139:1752–1760. - PubMed
-
- Caraty A, Smith JT, Lomet D, Ben Said S, Morrissey A, Cognie J, Doughton B, Baril G, Briant C, Clarke IJ. Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology. 2007;148:5258–5267. - PubMed
-
- Clarke IJ. Effector mechanisms of the hypothalamus that regulate the anterior pituitary gland. In: Unsicker K, editor. The Autonomic Nervous System. Harwood; London: 1996. pp. 45–88.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

