The aging male hypothalamic-pituitary-gonadal axis: pulsatility and feedback
- PMID: 18838102
- PMCID: PMC2662347
- DOI: 10.1016/j.mce.2008.09.005
The aging male hypothalamic-pituitary-gonadal axis: pulsatility and feedback
Abstract
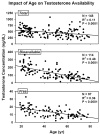
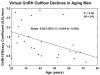
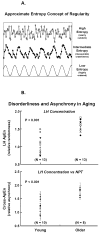
Aging results in insidious decremental changes in hypothalamic, pituitary and gonadal function. The foregoing three main anatomic loci of control are regulated by intermittent time-delayed signal exchange, principally via gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH) and testosterone/estradiol (Te/E(2)). A mathematical framework is required to embody these dynamics. The present review highlights integrative adaptations in the aging male hypothalamic-pituitary-gonadal axis, as assessed by recent objective ensemble models of the axis as a whole.
Figures








Similar articles
-
Hypothalamic-pituitary-gonadal axis in two men with aromatase deficiency: evidence that circulating estrogens are required at the hypothalamic level for the integrity of gonadotropin negative feedback.Eur J Endocrinol. 2006 Oct;155(4):513-22. doi: 10.1530/eje.1.02254. Eur J Endocrinol. 2006. PMID: 16990650
-
Regulation of complex pulsatile and rhythmic neuroendocrine systems: the male gonadal axis as a prototype.Prog Brain Res. 2010;181:79-110. doi: 10.1016/S0079-6123(08)81006-0. Prog Brain Res. 2010. PMID: 20478434 Review.
-
Two-week pulsatile gonadotropin releasing hormone infusion unmasks dual (hypothalamic and Leydig cell) defects in the healthy aging male gonadotropic axis.Eur J Endocrinol. 1999 Sep;141(3):257-66. doi: 10.1530/eje.0.1410257. Eur J Endocrinol. 1999. PMID: 10474123 Clinical Trial.
-
Central aspects of systemic oestradiol negative- and positive-feedback on the reproductive neuroendocrine system.J Neuroendocrinol. 2020 Jan;32(1):e12724. doi: 10.1111/jne.12724. Epub 2019 May 23. J Neuroendocrinol. 2020. PMID: 31054210 Free PMC article. Review.
-
Effects of aging on the hypothalamic-hypophyseal-gonadal axis in female rats.Fertil Steril. 1977 Dec;28(12):1365-70. doi: 10.1016/s0015-0282(16)42986-9. Fertil Steril. 1977. PMID: 201506
Cited by
-
Why men age faster but reproduce longer than women: mTOR and evolutionary perspectives.Aging (Albany NY). 2010 May;2(5):265-73. doi: 10.18632/aging.100149. Aging (Albany NY). 2010. PMID: 20519781 Free PMC article.
-
Improved early postnatal nutrition and its effect on histomorphological parameters in the testes of Sanjabi ram lambs.Trop Anim Health Prod. 2019 Jul;51(6):1539-1544. doi: 10.1007/s11250-019-01842-0. Epub 2019 Feb 13. Trop Anim Health Prod. 2019. PMID: 30759296
-
Feedback on LH in Testosterone-Clamped Men Depends on the Mode of Testosterone Administration and Body Composition.J Endocr Soc. 2018 Nov 23;3(1):235-249. doi: 10.1210/js.2018-00317. eCollection 2019 Jan 1. J Endocr Soc. 2018. PMID: 30623162 Free PMC article.
-
Short-Acting Testosterone: More Physiologic?Front Endocrinol (Lausanne). 2020 Sep 30;11:572465. doi: 10.3389/fendo.2020.572465. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33117287 Free PMC article. No abstract available.
-
Effects of Heat Stress-Induced Sex Hormone Dysregulation on Reproduction and Growth in Male Adolescents and Beneficial Foods.Nutrients. 2024 Sep 8;16(17):3032. doi: 10.3390/nu16173032. Nutrients. 2024. PMID: 39275346 Free PMC article. Review.
References
-
- Akingbemi BT, Ge R, Rosenfeld CS, Newton LG, Hardy DO, Catterall JF, Lubahn DB, Korach KS, Hardy MP. Estrogen receptor-alpha gene deficiency enhances androgen biosynthesis in the mouse Leydig cell. Endocrinol. 2003;144:84–93. - PubMed
-
- Cao L, Leers-Sucheta S, Azhar S. Aging alters the functional expression of enzymatic and non-enzymatic anti-oxidant defense systems in testicular rat Leydig cells. J Steroid Biochem Mol Biol. 2004;88:61–67. - PubMed
-
- Clarke IJ, Cummins JT. The significance of small pulses of gonadotropin-releasing hormone. J Endocrinol. 1987;113:413–418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

