Receptor tyrosine phosphatase beta (RPTPbeta) activity and signaling are attenuated by glycosylation and subsequent cell surface galectin-1 binding
- PMID: 18838383
- PMCID: PMC2586282
- DOI: 10.1074/jbc.M803646200
Receptor tyrosine phosphatase beta (RPTPbeta) activity and signaling are attenuated by glycosylation and subsequent cell surface galectin-1 binding
Abstract
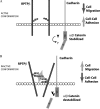
O-Mannosyl-linked glycosylation is abundant within the central nervous system, yet very few glycoproteins with this glycan modification have been identified. Congenital diseases with significant neurological defects arise from inactivating mutations found within the glycosyltransferases that act early in the O-mannosyl glycosylation pathway. The N-acetylglucosaminyltransferase known as GnT-Vb or -IX is highly expressed in brain and branches O-mannosyl-linked glycans. Our results using SH-SY5Y neuroblastoma cells indicate that GnT-Vb activity promotes the addition of the O-mannosyl-linked HNK-1 modification found on the developmentally regulated and neuron-specific receptor protein-tyrosine phosphatase beta (RPTPbeta). These changes in glycosylation accompany decreased cell-cell adhesion and increased rates of migration on laminin. In addition, we show that expression of GnT-Vb promotes its dimerization and inhibits RPTPbeta intrinsic phosphatase activity, resulting in higher levels of phosphorylated beta-catenin, suggesting a mechanism by which GnT-Vb glycosylation couples to changes in cell adhesion. GnT-Vb-mediated glycosylation of RPTPbeta promotes galectin-1 binding and RPTPbeta levels of retention on the cell surface. N-Acetyllactosamine, but not sucrose, treatment of cells results in decreased RPTP retention, showing that galectin-1 binding contributes to the increased retention after GnT-Vb expression. These results place GnT-Vb as a regulator of RPTPbeta signaling that influences cell-cell and cell-matrix interactions in the developing nervous system.
Figures


References
-
- Breen, K. C., Coughlan, C. M., and Hayes, F. D. (1998) Mol. Neurobiol. 16 163–220 - PubMed
-
- Schwarting, G. A., Jungalwala, F. B., Chou, D. K., Boyer, A. M., and Yamamoto, M. (1987) Dev. Biol. 120 65–76 - PubMed
-
- Bronner-Fraser, M. (1986) Dev. Biol. 115 44–55 - PubMed
-
- Abo, T., and Balch, C. M. (1981) J. Immunol. 127 1024–1029 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

