Analysis of the specificity of Panton-Valentine leucocidin and gamma-hemolysin F component binding
- PMID: 18838523
- PMCID: PMC2612235
- DOI: 10.1128/IAI.00402-08
Analysis of the specificity of Panton-Valentine leucocidin and gamma-hemolysin F component binding
Abstract
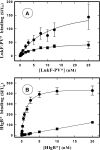
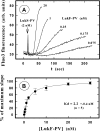
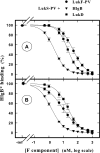
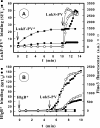
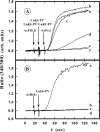
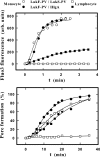
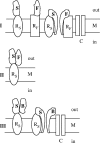
In this study, the binding of F components of the staphylococcal bicomponent leukotoxins Panton-Valentine leucocidin (LukF-PV) and gamma-hemolysin (HlgB) on polymorphonuclear neutrophils (PMNs), monocytes, and lymphocytes was determined using labeled mutants and flow cytometry. Leukotoxin activity was evaluated by measuring Ca(2+) entry or pore formation using spectrofluorometry or flow cytometry. Although HlgB had no affinity for cells in the absence of an S component, LukF-PV had high affinity for PMNs (dissociation constant [K(d)], 6.2 +/- 1.9 nM; n = 8), monocytes (K(d), 2.8 +/- 0.8 nM; n = 7), and lymphocytes (K(d), 1.2 +/- 0.2 nM; n = 7). Specific binding of HlgB was observed only after addition of LukS-PV on PMNs (K(d), 1.1 +/- 0.2 nM; n = 4) and monocytes (K(d), 0.84 +/- 0.31 nM; n = 4) or after addition of HlgC on PMNs, monocytes, and lymphocytes. Addition of LukS-PV or HlgC induced a second specific binding of LukF-PV on PMNs. HlgB and LukD competed only with LukF-PV molecules bound after addition of LukS-PV. LukF-PV and LukD competed with HlgB in the presence of LukS-PV on PMNs and monocytes. Use of antibodies and comparisons between binding and activity time courses showed that the LukF-PV molecules that bound to target cells before addition of LukS-PV were the only LukF-PV molecules responsible for Ca(2+) entry and pore formation. In contrast, the active HlgB molecules were the HlgB molecules bound after addition of LukS-PV. In conclusion, LukF-PV must be linked to LukS-PV and to a binding site of the membrane to have toxin activity.
Figures


References
-
- Baba Moussa, L., S. Werner, D. A. Colin, L. Mourey, J. D. Pédelacq, J. P. Samama, A. Sanni, H. Monteil, and G. Prévost. 1999. Discoupling the Ca2+-activation from the pore-forming function of the bi-component Panton-Valentine leucocidin in human PMNs. FEBS Lett. 461280-286. - PubMed
-
- Bocchini, C. E., K. G. Hulten, E. O. Mason, Jr., B. E. Gonzalez, W. A. Hammerman, and S. L. Kaplan. 2006. Panton-Valentine leukocidin genes are associated with enhanced inflammatory response and local disease in acute hematogenous Staphylococcus aureus ostomyelitis in children. Pediatrics 117433-440. - PubMed
-
- Boyle-Vavra, S., and R. S. Daum. 2007. Community-acquired methicillin-resistant Staphylococcus aureus: the role of Panton-Valentine leukocidin. Lab. Investig. 873-9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

