Aquaporin-4-binding autoantibodies in patients with neuromyelitis optica impair glutamate transport by down-regulating EAAT2
- PMID: 18838545
- PMCID: PMC2571922
- DOI: 10.1084/jem.20081241
Aquaporin-4-binding autoantibodies in patients with neuromyelitis optica impair glutamate transport by down-regulating EAAT2
Abstract
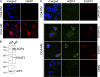
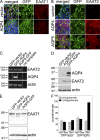
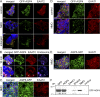
Neuromyelitis optica (NMO)-immunoglobulin G (IgG) is a clinically validated serum biomarker that distinguishes relapsing central nervous system (CNS) inflammatory demyelinating disorders related to NMO from multiple sclerosis. This autoantibody targets astrocytic aquaporin-4 (AQP4) water channels. Clinical, radiological, and immunopathological data suggest that NMO-IgG might be pathogenic. Characteristic CNS lesions exhibit selective depletion of AQP4, with and without associated myelin loss; focal vasculocentric deposits of IgG, IgM, and complement; prominent edema; and inflammation. The effect of NMO-IgG on astrocytes has not been studied. In this study, we demonstrate that exposure to NMO patient serum and active complement compromises the membrane integrity of CNS-derived astrocytes. Without complement, astrocytic membranes remain intact, but AQP4 is endocytosed with concomitant loss of Na(+)-dependent glutamate transport and loss of the excitatory amino acid transporter 2 (EAAT2) . Our data suggest that EAAT2 and AQP4 exist in astrocytic membranes as a macromolecular complex. Transport-competent EAAT2 protein is up-regulated in differentiating astrocyte progenitors and in nonneural cells expressing AQP4 transgenically. Marked reduction of EAAT2 in AQP4-deficient regions of NMO patient spinal cord lesions supports our immunocytochemical and immunoprecipitation data. Thus, binding of NMO-IgG to astrocytic AQP4 initiates several potentially neuropathogenic mechanisms: complement activation, AQP4 and EAAT2 down-regulation, and disruption of glutamate homeostasis.
Figures


References
-
- Wingerchuk, D.M., V.A. Lennon, C.F. Lucchinetti, S.J. Pittock, and B.G. Weinshenker. 2007. The spectrum of neuromyelitis optica. Lancet Neurol. 6:805–815. - PubMed
-
- Nakamura, M., I. Miyazawa, K. Fujihara, I. Nakashima, T. Misu, S. Watanabe, T. Takahashi, and Y. Itoyama. 2008. Preferential spinal central gray matter involvement in neuromyelitis optica. An MRI study. J. Neurol. 255:163–170. - PubMed
-
- Roemer, S.F., J.E. Parisi, V.A. Lennon, E.E. Benarroch, H. Lassmann, W. Bruck, R.N. Mandler, B.G. Weinshenker, S.J. Pittock, D.M. Wingerchuk, and C.F. Lucchinetti. 2007. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 130:1194–1205. - PubMed

