Macaques vaccinated with live-attenuated SIV control replication of heterologous virus
- PMID: 18838548
- PMCID: PMC2571929
- DOI: 10.1084/jem.20081524
Macaques vaccinated with live-attenuated SIV control replication of heterologous virus
Abstract
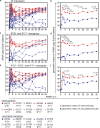
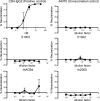
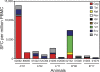
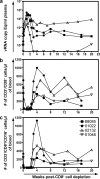
An effective AIDS vaccine will need to protect against globally diverse isolates of HIV. To address this issue in macaques, we administered a live-attenuated simian immunodeficiency virus (SIV) vaccine and challenged with a highly pathogenic heterologous isolate. Vaccinees reduced viral replication by approximately 2 logs between weeks 2-32 (P < or = 0.049) postchallenge. Remarkably, vaccinees expressing MHC-I (MHC class I) alleles previously associated with viral control completely suppressed acute phase replication of the challenge virus, implicating CD8(+) T cells in this control. Furthermore, transient depletion of peripheral CD8(+) lymphocytes in four vaccinees during the chronic phase resulted in an increase in virus replication. In two of these animals, the recrudescent virus population contained only the vaccine strain and not the challenge virus. Alarmingly, however, we found evidence of recombinant viruses emerging in some of the vaccinated animals. This finding argues strongly against an attenuated virus vaccine as a solution to the AIDS epidemic. On a more positive note, our results suggest that MHC-I-restricted CD8(+) T cells contribute to the protection induced by the live-attenuated SIV vaccine and demonstrate that vaccine-induced CD8(+) T cell responses can control replication of heterologous challenge viruses.
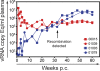
Figures


References
-
- Gaschen, B., J. Taylor, K. Yusim, B. Foley, F. Gao, D. Lang, V. Novitsky, B. Haynes, B.H. Hahn, T. Bhattacharya, and B. Korber. 2002. Diversity considerations in HIV-1 vaccine selection. Science. 296:2354–2360. - PubMed
-
- Korber, B., M. Muldoon, J. Theiler, F. Gao, R. Gupta, A. Lapedes, B.H. Hahn, S. Wolinsky, and T. Bhattacharya. 2000. Timing the ancestor of the HIV-1 pandemic strains. Science. 288:1789–1796. - PubMed
-
- Robertson, D.L., P.M. Sharp, F.E. McCutchan, and B.H. Hahn. 1995. Recombination in HIV-1. Nature. 374:124–126. - PubMed
-
- Heeney, J.L., A.G. Dalgleish, and R.A. Weiss. 2006. Origins of HIV and the evolution of resistance to AIDS. Science. 313:462–466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

