Minimal pharmacokinetic interaction between the human immunodeficiency virus nonnucleoside reverse transcriptase inhibitor etravirine and the integrase inhibitor raltegravir in healthy subjects
- PMID: 18838586
- PMCID: PMC2592888
- DOI: 10.1128/AAC.00487-08
Minimal pharmacokinetic interaction between the human immunodeficiency virus nonnucleoside reverse transcriptase inhibitor etravirine and the integrase inhibitor raltegravir in healthy subjects
Abstract
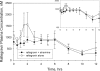
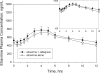
Etravirine, a next-generation nonnucleoside reverse transcriptase inhibitor, and raltegravir, an integrase strand transfer inhibitor, have separately demonstrated potent activity in treatment-experienced, human immunodeficiency virus (HIV)-infected patients. An open-label, sequential, three-period study with healthy, HIV-seronegative subjects was conducted to assess the two-way interaction between etravirine and raltegravir for potential coadministration to HIV-infected patients. In period 1, 19 subjects were administered 400 mg raltegravir every 12 h (q12 h) for 4 days, followed by a 4-day washout; in period 2, subjects were administered 200 mg etravirine q12 h for 8 days; and in period 3, subjects were coadministered 400 mg raltegravir and 200 mg etravirine q12 h for 4 days. There was no washout between periods 2 and 3. Doses were administered with a moderate-fat meal. Etravirine had only modest effects on the pharmacokinetics of raltegravir, while raltegravir had no clinically meaningful effect on the pharmacokinetics of etravirine. For raltegravir coadministered with etravirine relative to raltegravir alone, the geometric mean ratio (GMR) and 90% confidence interval (CI) were 0.90 and 0.68 to 1.18, respectively, for the area under the concentration curve from 0 to 12 h (AUC(0-12)), 0.89 and 0.68 to 1.15, respectively, for the maximum concentration of drug in serum (C(max)), and 0.66 and 0.34 to 1.26, respectively, for the trough drug concentration (C(12)); the GMR (90% CI) for etravirine coadministered with raltegravir relative to etravirine alone was 1.10 (1.03, 1.16) for AUC(0-12), 1.04 (0.97, 1.12) for C(max), and 1.17 (1.10, 1.26) for C(12). All drug-related adverse clinical experiences were mild and generally transient in nature. No grade 3 or 4 adverse experiences or discontinuations due to adverse experiences occurred. Coadministration of etravirine and raltegravir was generally well tolerated; the data suggest that no dose adjustment for either drug is necessary.
Figures
Similar articles
-
Minimal effects of ritonavir and efavirenz on the pharmacokinetics of raltegravir.Antimicrob Agents Chemother. 2008 Dec;52(12):4338-43. doi: 10.1128/AAC.01543-07. Epub 2008 Oct 6. Antimicrob Agents Chemother. 2008. PMID: 18838589 Free PMC article. Clinical Trial.
-
Lack of a significant drug interaction between raltegravir and tenofovir.Antimicrob Agents Chemother. 2008 Sep;52(9):3253-8. doi: 10.1128/AAC.00005-08. Epub 2008 Jul 14. Antimicrob Agents Chemother. 2008. PMID: 18625763 Free PMC article. Clinical Trial.
-
Raltegravir dosage adjustment in HIV-infected patients receiving etravirine.Am J Health Syst Pharm. 2011 Nov 1;68(21):2049-54. doi: 10.2146/ajhp110083. Am J Health Syst Pharm. 2011. PMID: 22011983
-
Raltegravir: the first HIV integrase inhibitor.Clin Ther. 2008 Oct;30(10):1747-65. doi: 10.1016/j.clinthera.2008.10.012. Clin Ther. 2008. PMID: 19014832 Review.
-
Etravirine, a next-generation nonnucleoside reverse-transcriptase inhibitor.Clin Infect Dis. 2009 Apr 15;48(8):1123-8. doi: 10.1086/597469. Clin Infect Dis. 2009. PMID: 19275497 Review.
Cited by
-
Drug-drug interactions with raltegravir.Eur J Med Res. 2009 Nov 24;14 Suppl 3(Suppl 3):17-21. doi: 10.1186/2047-783x-14-s3-17. Eur J Med Res. 2009. PMID: 19959412 Free PMC article. Review.
-
Long-term efficacy and safety of raltegravir in the management of HIV infection.Infect Drug Resist. 2014 Mar 18;7:73-84. doi: 10.2147/IDR.S40168. eCollection 2014. Infect Drug Resist. 2014. PMID: 24672249 Free PMC article. Review.
-
Effect of tipranavir-ritonavir on pharmacokinetics of raltegravir.Antimicrob Agents Chemother. 2009 Jul;53(7):2752-5. doi: 10.1128/AAC.01486-08. Epub 2009 Apr 27. Antimicrob Agents Chemother. 2009. PMID: 19398643 Free PMC article. Clinical Trial.
-
Role of raltegravir in the management of HIV-1 infection.HIV AIDS (Auckl). 2011;3:81-92. doi: 10.2147/HIV.S13985. Epub 2011 Jul 15. HIV AIDS (Auckl). 2011. PMID: 22096410 Free PMC article.
-
Population pharmacokinetic analysis and pharmacogenetics of raltegravir in HIV-positive and healthy individuals.Antimicrob Agents Chemother. 2012 Jun;56(6):2959-66. doi: 10.1128/AAC.05424-11. Epub 2012 Feb 27. Antimicrob Agents Chemother. 2012. PMID: 22371894 Free PMC article.
References
-
- Andries, K., H. Azijn, T. Thielemans, D. Ludovici, M. Kukla, J. Heeres, P. Janssen, B. De Corte, J. Vingerhoets, R. Pauwels, and M. P. de Béthune. 2004. TMC125, a novel next-generation nonnucleoside reverse transcriptase inhibitor active against nonnucleoside reverse transcriptase inhibitor-resistant human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 48:4680-4686. - PMC - PubMed
-
- Asante-Appiah, E., and A. M. Skalka. 1999. Integrase: structural organization, conformational changes, and catalysis. Adv. Virus Res. 52:351-369. - PubMed
-
- D'Souza, V., and M. F. Summers. 2005. How retroviruses select their genomes. Nat. Rev. Microbiol. 3:643-655. - PubMed
-
- Esposito, D., and R. Craigie. 1999. HIV integrase structure and function. Adv. Virus Res. 52:319-333. - PubMed
-
- Iwamoto, M., K. Kassahun, M. D. Troyer, W. D. Hanley, P. Lu, A. Rhoton, A. S. Petry, K. Ghosh, E. Mangin, E. P. Denoia, L. A. Wenning, J. A. Stone, K. M. Gottesdiener, and J. A. Wagner. 2008. Lack of a pharmacokinetic effect of raltegravir on midazolam: in vitro/in vivo correlation. J. Clin. Pharmacol. 48:209-214. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

