Stool DNA and occult blood testing for screen detection of colorectal neoplasia
- PMID: 18838724
- PMCID: PMC4016975
- DOI: 10.7326/0003-4819-149-7-200810070-00004
Stool DNA and occult blood testing for screen detection of colorectal neoplasia
Abstract
Background: Stool DNA testing is a new approach to colorectal cancer detection. Few data are available from the screening setting.
Objective: To compare stool DNA and fecal blood testing for detection of screen-relevant neoplasia (curable-stage cancer, high-grade dysplasia, or adenomas >1 cm).
Design: Blinded, multicenter, cross-sectional study.
Setting: Communities surrounding 22 participating academic and regional health care systems in the United States.
Participants: 4482 average-risk adults.
Measurements: Fecal blood and DNA markers. Participants collected 3 stools, smeared fecal blood test cards and used same-day shipment to a central facility. Fecal blood cards (Hemoccult and HemoccultSensa, Beckman Coulter, Fullerton, California) were tested on 3 stools and DNA assays on 1 stool per patient. Stool DNA test 1 (SDT-1) was a precommercial 23-marker assay, and a novel test (SDT-2) targeted 3 broadly informative markers. The criterion standard was colonoscopy.
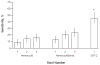
Results: Sensitivity for screen-relevant neoplasms was 20% by SDT-1, 11% by Hemoccult (P = 0.020), 21% by HemoccultSensa (P = 0.80); sensitivity for cancer plus high-grade dysplasia did not differ among tests. Specificity was 96% by SDT-1, compared with 98% by Hemoccult (P < 0.001) and 97% by HemoccultSensa (P = 0.20). Stool DNA test 2 detected 46% of screen-relevant neoplasms, compared with 16% by Hemoccult (P < 0.001) and 24% by HemoccultSensa (P < 0.001). Stool DNA test 2 detected 46% of adenomas 1 cm or larger, compared with 10% by Hemoccult (P < 0.001) and 17% by HemoccultSensa (P < 0.001). Among colonoscopically normal patients, the positivity rate was 16% with SDT-2, compared with 4% with Hemoccult (P = 0.010) and 5% with HemoccultSensa (P = 0.030).
Limitations: Stool DNA test 2 was not performed on all subsets of patients without screen-relevant neoplasms. Stools were collected without preservative, which reduced detection of some DNA markers.
Conclusion: Stool DNA test 1 provides no improvement over HemoccultSensa for detection of screen-relevant neoplasms. Stool DNA test 2 detects significantly more neoplasms than does Hemoccult or HemoccultSensa, but with more positive results in colonoscopically normal patients. Higher sensitivity of SDT-2 was particularly apparent for adenomas.
Conflict of interest statement
Figures
Comment in
-
Stool DNA testing and colon cancer prevention: another step forward.Ann Intern Med. 2008 Oct 7;149(7):509-10. doi: 10.7326/0003-4819-149-7-200810070-00013. Ann Intern Med. 2008. PMID: 18838731 No abstract available.
Summary for patients in
-
Summaries for patients. Stool DNA and occult blood testing to screen for colorectal neoplasia.Ann Intern Med. 2008 Oct 7;149(7):I20. doi: 10.7326/0003-4819-149-7-200810070-00001. Ann Intern Med. 2008. PMID: 18838720 No abstract available.
References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. - PubMed
-
- Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–71. - PubMed
-
- Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–7. - PubMed
-
- Kronborg O, Jørgensen OD, Fenger C, Rasmussen M. Randomized study of biennial screening with a faecal occult blood test: results after nine screening rounds. Scand J Gastroenterol. 2004;39:846–51. - PubMed
-
- Winawer SJ, Flehinger BJ, Schottenfeld D, Miller DG. Screening for colorectal cancer with fecal occult blood testing and sigmoidoscopy. J Natl Cancer Inst. 1993;85:1311–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
