Highly active antiretroviral therapy versus zidovudine/nevirapine effects on early breast milk HIV type-1 Rna: a phase II randomized clinical trial
- PMID: 18839781
- PMCID: PMC2859833
Highly active antiretroviral therapy versus zidovudine/nevirapine effects on early breast milk HIV type-1 Rna: a phase II randomized clinical trial
Abstract
Background: Defining the effect of antiretroviral regimens on breast milk HIV type-1 (HIV-1) levels is useful to inform the rational design of strategies to decrease perinatal HIV-1 transmission.
Methods: Pregnant HIV-1 seropositive women (CD4+ T-cell count >250 and <500 cells/mm3) electing to breastfeed in Nairobi, Kenya were randomized to highly active antiretroviral therapy (HAART; zidovudine [ZDV], lamivudine and nevirapine [NVP]) during pregnancy and 6 months post-partum or to short-course ZDV plus single-dose NVP (ZDV/NVP). Breast milk samples were collected two to three times per week in the first month post-partum.
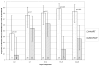
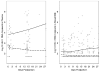
Results: Between November 2003 and April 2006, 444 breast milk samples were collected from 58 randomized women during the first month after delivery. Between 3 and 14 days post-partum, women in the HAART and ZDV/NVP arms had a similar prevalence of undetectable breast milk HIV-1 RNA. From 15 to 28 days post-partum, women in the HAART arm had significantly lower levels of breast milk HIV-1 RNA than women randomized to ZDV/NVP (1.7 log10 copies/ml [limit of detection] versus >2.10 log10 copies/ml, P<0.001). In contrast to breast milk HIV-1 RNA, suppression of plasma HIV-1 RNA during the neonatal period was consistently several log10 greater in the HAART arm compared with the ZDV/NVP arm.
Conclusions: HAART resulted in lower breast milk HIV-1 RNA than ZDV/NVP; however, ZDV/NVP yielded comparable breast milk HIV-1 RNA levels in the first 2 weeks post-partum. Breast milk HIV-1 RNA remained suppressed in the ZDV/NVP arm despite increased plasma HIV-1 levels, which might reflect local drug effects or compartmentalization.
Trial registration: ClinicalTrials.gov NCT00167674.
Conflict of interest statement
None of the authors has a major conflict of interest in this study.
Figures
References
-
- Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, Mwatha A, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–1174. - PubMed
-
- WHO. HIV and infant feeding technical consultation, October 25-27, 2006. Geneva: World Health Organization; 2006.
-
- Sinkala M, Kuhn L, Kankasa C, Kasonde P, Vwalika C, Mwiya M, et al. No Benefit of Early Cessation of Breastfeeding at 4 Months on HIV-free Survival of Infants Born to HIV-infected Mothers in Zambia: The Zambia Exclusive Breastfeeding Study. Fourteenth Conference on Retroviruses and Opportunistic Infections; Los Angeles. 2007.
-
- Thior I, Lockman S, Smeaton LM, Shapiro RL, Wester C, Heymann SJ, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: a randomized trial: the Mashi Study. JAMA. 2006;296:794–805. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous