Changes in water structure induced by the guanidinium cation and implications for protein denaturation
- PMID: 18839935
- PMCID: PMC2646201
- DOI: 10.1021/jp8058239
Changes in water structure induced by the guanidinium cation and implications for protein denaturation
Abstract
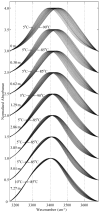
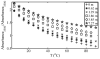
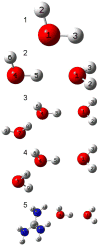
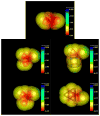
The effect of the guanidinium cation on the hydrogen bonding strength of water was analyzed using temperature-excursion Fourier transform infrared spectra of the OH stretching vibration in 5% H 2O/95% D 2O solutions containing a range of different guanidine-HCl and guanidine-HBr concentrations. Our findings indicate that the guanidinium cation causes the water H-bonds in solution to become more linear than those found in bulk water, and that it also inhibits the response of the H-bond network to increased temperature. Quantum chemical calculations also reveal that guanidinium affects both the charge distribution on water molecules directly H-bonded to it as well as the OH stretch frequency of H-bonds in which that water molecule is the donor. The implications of our findings to hydrophobic solvation and protein denaturation are discussed.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

