Chlorination increases the persistence of semiquinone free radicals derived from polychlorinated biphenyl hydroquinones and quinones
- PMID: 18839991
- PMCID: PMC2629131
- DOI: 10.1021/jo801397g
Chlorination increases the persistence of semiquinone free radicals derived from polychlorinated biphenyl hydroquinones and quinones
Abstract
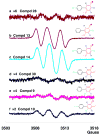
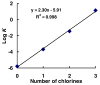
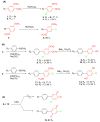
Polychlorinated biphenyls (PCBs) comprise a group of persistent organic pollutants that differ significantly in their physicochemical properties, their persistence, and their biological activities. They can be metabolized via hydroxylated and dihydroxylated metabolites to PCB quinone intermediates. We have recently demonstrated that both dihydroxy PCBs and PCB quinones can form semiquinone radicals (SQ(*-)) in vitro. These semiquinone radicals are reactive intermediates that have been implicated in the toxicity of lower chlorinated PCB congeners. Here we describe the synthesis of selected PCB metabolites with differing degrees of chlorination on the oxygenated phenyl ring, e.g., 4,4'-dichloro-biphenyl-2,5-diol, 3,6,4'-trichloro-biphenyl-2,5-diol, 3,4,6,-trichloro-biphenyl-2,5-diol, and their corresponding quinones. In addition, two chlorinated o-hydroquinones were prepared, 6-chloro-biphenyl-3,4-diol and 6,4'-dichloro-biphenyl-3,4-diol. These PCB (hydro-)quinones readily react with oxygen or via comproportionation to yield the corresponding semiquinone free radicals, as detected by electron paramagnetic resonance spectroscopy (EPR alias ESR). The greater the number of chlorines on the (hydro-)quinone (oxygenated) ring, the higher the steady-state level of the resulting semiquinone radical at near neutral pH.
Figures




Similar articles
-
Semiquinone radicals from oxygenated polychlorinated biphenyls: electron paramagnetic resonance studies.Chem Res Toxicol. 2008 Jul;21(7):1359-67. doi: 10.1021/tx8000175. Epub 2008 Jun 13. Chem Res Toxicol. 2008. PMID: 18549251 Free PMC article.
-
Nonenzymatic displacement of chlorine and formation of free radicals upon the reaction of glutathione with PCB quinones.Proc Natl Acad Sci U S A. 2009 Jun 16;106(24):9725-30. doi: 10.1073/pnas.0810352106. Epub 2009 Jun 2. Proc Natl Acad Sci U S A. 2009. PMID: 19497881 Free PMC article.
-
Breaking the dogma: PCB-derived semiquinone free radicals do not form covalent adducts with DNA, GSH, and amino acids.Environ Sci Pollut Res Int. 2016 Feb;23(3):2138-47. doi: 10.1007/s11356-015-5007-4. Epub 2015 Sep 23. Environ Sci Pollut Res Int. 2016. PMID: 26396011 Free PMC article.
-
Polychlorinated biphenyls and human health.Int J Occup Med Environ Health. 1998;11(4):291-303. Int J Occup Med Environ Health. 1998. PMID: 10028197 Review.
-
Microbial and chemically induced reductive dechlorination of polychlorinated biphenyls in the environment.Environ Sci Pollut Res Int. 2025 Jan;32(5):2167-2181. doi: 10.1007/s11356-024-35831-0. Epub 2025 Jan 7. Environ Sci Pollut Res Int. 2025. PMID: 39762530 Review.
Cited by
-
A new player in environmentally induced oxidative stress: polychlorinated biphenyl congener, 3,3'-dichlorobiphenyl (PCB11).Toxicol Sci. 2013 Nov;136(1):39-50. doi: 10.1093/toxsci/kft186. Epub 2013 Aug 31. Toxicol Sci. 2013. PMID: 23997111 Free PMC article.
-
Polychlorinated biphenyl induced ROS signaling delays the entry of quiescent human breast epithelial cells into the proliferative cycle.Free Radic Biol Med. 2010 Jul 1;49(1):40-9. doi: 10.1016/j.freeradbiomed.2010.03.012. Epub 2010 Mar 20. Free Radic Biol Med. 2010. PMID: 20307652 Free PMC article.
-
Modelling changes in glutathione homeostasis as a function of quinone redox metabolism.Sci Rep. 2019 Apr 19;9(1):6333. doi: 10.1038/s41598-019-42799-2. Sci Rep. 2019. PMID: 31004119 Free PMC article.
-
Crystal structure and density functional theory studies of toxic quinone metabolites of polychlorinated biphenyls.Chemosphere. 2011 Oct;85(3):386-92. doi: 10.1016/j.chemosphere.2011.07.004. Epub 2011 Aug 6. Chemosphere. 2011. PMID: 21824639 Free PMC article.
-
Catalytic Reactions and Energy Conservation in the Cytochrome bc1 and b6f Complexes of Energy-Transducing Membranes.Chem Rev. 2021 Feb 24;121(4):2020-2108. doi: 10.1021/acs.chemrev.0c00712. Epub 2021 Jan 19. Chem Rev. 2021. PMID: 33464892 Free PMC article. Review.
References
-
- Robertson LW, Hansen LG. Recent advances in the environmental toxicology and health effects of PCBs. University Press of Kentucky; Lexington: 2001.
-
- Hansen LG. The ortho side of PCBs: Occurrence and disposition. Kluwer Academic Publishers; Boston: 1999.
-
- Kodavanti PRS. In: Molecular Neurotoxicology: environmental agents and transcription-transduction coupling. Zawia NH, editor. CRC press; Boca Roton, FL: 2004. pp. 151–182.
-
- Silberhorn E, Glauert HP, Robertson LW. Crit Rev Toxicol. 1990;20:439–496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

