Chlorination increases the persistence of semiquinone free radicals derived from polychlorinated biphenyl hydroquinones and quinones
- PMID: 18839991
- PMCID: PMC2629131
- DOI: 10.1021/jo801397g
Chlorination increases the persistence of semiquinone free radicals derived from polychlorinated biphenyl hydroquinones and quinones
Abstract
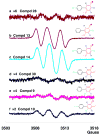
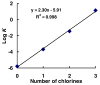
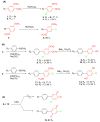
Polychlorinated biphenyls (PCBs) comprise a group of persistent organic pollutants that differ significantly in their physicochemical properties, their persistence, and their biological activities. They can be metabolized via hydroxylated and dihydroxylated metabolites to PCB quinone intermediates. We have recently demonstrated that both dihydroxy PCBs and PCB quinones can form semiquinone radicals (SQ(*-)) in vitro. These semiquinone radicals are reactive intermediates that have been implicated in the toxicity of lower chlorinated PCB congeners. Here we describe the synthesis of selected PCB metabolites with differing degrees of chlorination on the oxygenated phenyl ring, e.g., 4,4'-dichloro-biphenyl-2,5-diol, 3,6,4'-trichloro-biphenyl-2,5-diol, 3,4,6,-trichloro-biphenyl-2,5-diol, and their corresponding quinones. In addition, two chlorinated o-hydroquinones were prepared, 6-chloro-biphenyl-3,4-diol and 6,4'-dichloro-biphenyl-3,4-diol. These PCB (hydro-)quinones readily react with oxygen or via comproportionation to yield the corresponding semiquinone free radicals, as detected by electron paramagnetic resonance spectroscopy (EPR alias ESR). The greater the number of chlorines on the (hydro-)quinone (oxygenated) ring, the higher the steady-state level of the resulting semiquinone radical at near neutral pH.
Figures




References
-
- Robertson LW, Hansen LG. Recent advances in the environmental toxicology and health effects of PCBs. University Press of Kentucky; Lexington: 2001.
-
- Hansen LG. The ortho side of PCBs: Occurrence and disposition. Kluwer Academic Publishers; Boston: 1999.
-
- Kodavanti PRS. In: Molecular Neurotoxicology: environmental agents and transcription-transduction coupling. Zawia NH, editor. CRC press; Boca Roton, FL: 2004. pp. 151–182.
-
- Silberhorn E, Glauert HP, Robertson LW. Crit Rev Toxicol. 1990;20:439–496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

