The lipid messenger OEA links dietary fat intake to satiety
- PMID: 18840358
- PMCID: PMC2572640
- DOI: 10.1016/j.cmet.2008.08.005
The lipid messenger OEA links dietary fat intake to satiety
Abstract
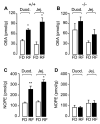
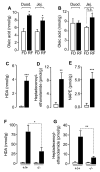
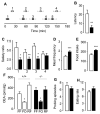
The association between fat consumption and obesity underscores the need to identify physiological signals that control fat intake. Previous studies have shown that feeding stimulates small-intestinal mucosal cells to produce the lipid messenger oleoylethanolamide (OEA) which, when administered as a drug, decreases meal frequency by engaging peroxisome proliferator-activated receptors-alpha (PPAR-alpha). Here, we report that duodenal infusion of fat stimulates OEA mobilization in the proximal small intestine, whereas infusion of protein or carbohydrate does not. OEA production utilizes dietary oleic acid as a substrate and is disrupted in mutant mice lacking the membrane fatty-acid transporter CD36. Targeted disruption of CD36 or PPAR-alpha abrogates the satiety response induced by fat. The results suggest that activation of small-intestinal OEA mobilization, enabled by CD36-mediated uptake of dietary oleic acid, serves as a molecular sensor linking fat ingestion to satiety.
Figures
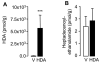
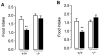
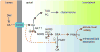
References
-
- Ahern GP. Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem. 2003;278:30429–30434. - PubMed
-
- Artmann A, Petersen G, Hellgren LI, Boberg J, Skonberg C, Nellemann C, Hansen SH, Hansen HS. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim Biophys Acta. 2008;1781:200–212. - PubMed
-
- Astarita G, Di Giacomo B, Gaetani S, Oveisi F, Compton TR, Rivara S, Tarzia G, Mor M, Piomelli D. Pharmacological characterization of hydrolysis-resistant analogs of oleoylethanolamide with potent anorexiant properties. J Pharmacol Exp Ther. 2006a;318:563–570. - PubMed
-
- Astarita G, Rourke BC, Andersen JB, Fu J, Kim JH, Bennett AF, Hicks JW, Piomelli D. Postprandial increase of oleoylethanolamide mobilization in small intestine of the Burmese python (Python molurus) Am J Physiol Regul Integr Comp Physiol. 2006b;290:R1407–1412. - PubMed
-
- Azzara AV, Sokolnicki JP, Schwartz GJ. Central melanocortin receptor agonist reduces spontaneous and scheduled meal size but does not augment duodenal preload-induced feeding inhibition. Physiol Behav. 2002;77:411–416. - PubMed
