A high-resolution computational atlas of the human hippocampus from postmortem magnetic resonance imaging at 9.4 T
- PMID: 18840532
- PMCID: PMC2650508
- DOI: 10.1016/j.neuroimage.2008.08.042
A high-resolution computational atlas of the human hippocampus from postmortem magnetic resonance imaging at 9.4 T
Abstract
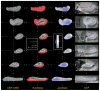
This paper describes the construction of a computational anatomical atlas of the human hippocampus. The atlas is derived from high-resolution 9.4 Tesla MRI of postmortem samples. The main subfields of the hippocampus (cornu ammonis fields CA1, CA2/3; the dentate gyrus; and the vestigial hippocampal sulcus) are labeled in the images manually using a combination of distinguishable image features and geometrical features. A synthetic average image is derived from the MRI of the samples using shape and intensity averaging in the diffeomorphic non-linear registration framework, and a consensus labeling of the template is generated. The agreement of the consensus labeling with manual labeling of each sample is measured, and the effect of aiding registration with landmarks and manually generated mask images is evaluated. The atlas is provided as an online resource with the aim of supporting subfield segmentation in emerging hippocampus imaging and image analysis techniques. An example application examining subfield-level hippocampal atrophy in temporal lobe epilepsy demonstrates the application of the atlas to in vivo studies.
Figures






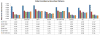





Similar articles
-
Histology-derived volumetric annotation of the human hippocampal subfields in postmortem MRI.Neuroimage. 2014 Jan 1;84:505-23. doi: 10.1016/j.neuroimage.2013.08.067. Epub 2013 Sep 12. Neuroimage. 2014. PMID: 24036353 Free PMC article.
-
Automatic hippocampus segmentation of 7.0 Tesla MR images by combining multiple atlases and auto-context models.Neuroimage. 2013 Dec;83:335-45. doi: 10.1016/j.neuroimage.2013.06.006. Epub 2013 Jun 11. Neuroimage. 2013. PMID: 23769921 Free PMC article.
-
Shape-based alignment of hippocampal subfields: evaluation in postmortem MRI.Med Image Comput Comput Assist Interv. 2008;11(Pt 1):510-7. doi: 10.1007/978-3-540-85988-8_61. Med Image Comput Comput Assist Interv. 2008. PMID: 18979785 Free PMC article.
-
Surface-based multi-template automated hippocampal segmentation: application to temporal lobe epilepsy.Med Image Anal. 2012 Oct;16(7):1445-55. doi: 10.1016/j.media.2012.04.008. Epub 2012 May 3. Med Image Anal. 2012. PMID: 22613821
-
Defining the human hippocampus in cerebral magnetic resonance images--an overview of current segmentation protocols.Neuroimage. 2009 Oct 1;47(4):1185-95. doi: 10.1016/j.neuroimage.2009.05.019. Epub 2009 May 15. Neuroimage. 2009. PMID: 19447182 Free PMC article. Review.
Cited by
-
A multimodal neuroimaging study of brain abnormalities and clinical correlates in post treatment Lyme disease.PLoS One. 2022 Oct 26;17(10):e0271425. doi: 10.1371/journal.pone.0271425. eCollection 2022. PLoS One. 2022. PMID: 36288329 Free PMC article.
-
Hippocampal CA1 apical neuropil atrophy and memory performance in Alzheimer's disease.Neuroimage. 2012 Oct 15;63(1):194-202. doi: 10.1016/j.neuroimage.2012.06.048. Epub 2012 Jul 3. Neuroimage. 2012. PMID: 22766164 Free PMC article.
-
A Diffeomorphic Vector Field Approach to Analyze the Thickness of the Hippocampus From 7 T MRI.IEEE Trans Biomed Eng. 2021 Feb;68(2):393-403. doi: 10.1109/TBME.2020.2999941. Epub 2021 Jan 20. IEEE Trans Biomed Eng. 2021. PMID: 32746019 Free PMC article.
-
Histology-derived volumetric annotation of the human hippocampal subfields in postmortem MRI.Neuroimage. 2014 Jan 1;84:505-23. doi: 10.1016/j.neuroimage.2013.08.067. Epub 2013 Sep 12. Neuroimage. 2014. PMID: 24036353 Free PMC article.
-
Morphological Changes of Amygdala in Turner Syndrome Patients.CNS Neurosci Ther. 2016 Mar;22(3):194-9. doi: 10.1111/cns.12482. Epub 2016 Jan 18. CNS Neurosci Ther. 2016. PMID: 26778543 Free PMC article.
References
-
- Amaral DG. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol. 1978;182(4 Pt 2):851–914. - PubMed
-
- Apostolova LG, Dinov ID, Dutton RA, Hayashi KM, Toga AW, Cummings JL, Thompson PM. 3d comparison of hippocampal atrophy in amnestic mild cognitive impairment and alzheimer’s disease. Brain. 2006;129(Pt 11):2867–2873. - PubMed
-
- Avants B, Gee JC. Geodesic estimation for large deformation anatomical shape averaging and interpolation. Neuroimage. 2004;23(Suppl 1):S139–S150. - PubMed
-
- Beg MF, Miller MI, Trouvé A, Younes L. Computing large deformation metric mappings via geodesic flows of diffeomorphisms. Int J Comput Vision. 2005;61(2):139–157.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

