The calpain small subunit regulates cell-substrate mechanical interactions during fibroblast migration
- PMID: 18840650
- PMCID: PMC3081789
- DOI: 10.1242/jcs.036152
The calpain small subunit regulates cell-substrate mechanical interactions during fibroblast migration
Abstract
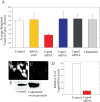
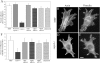
Cell migration involves the dynamic formation and release of cell-substrate adhesions, where the exertion and detection of mechanical forces take place. Members of the calpain family of calcium-dependent proteases are believed to have a central role in these processes, possibly through the regulation of focal adhesion dynamics. The ubiquitous calpains, calpain 1 (mu-calpain) and calpain 2 (m-calpain), are heterodimers consisting of large catalytic subunits encoded by the Capn1 and Capn2 genes, respectively, and the small regulatory subunit encoded by Capn4. We have examined the role of the calpain regulatory small subunit in traction force production and mechanosensing during cell migration. Capn4-deficient or rescued cells were plated on flexible polyacrylamide substrates, for both the detection of traction forces and the application of mechanical stimuli. The total force output of Capn4-deficient cells was approximately 75% lower than that of rescued cells and the forces were more randomly distributed and less dynamic in Capn4-deficient cells than in rescued cells. Furthermore, Capn4-deficient cells were less adhesive than wild-type cells and they also failed to respond to mechanical stimulations by pushing or pulling the flexible substrate, or by engaging dorsal receptors to the extracellular matrix. Surprisingly, fibroblasts deficient in calpain 1 or calpain 2 upon siRNA-mediated knockdown of Capn1 or Capn2, respectively, did not show the same defects in force production or adhesion, although they also failed to respond to mechanical stimulation. Interestingly, stress fibers were aberrant and also contained fewer colocalised vinculin-containing adhesions in Capn4-deficient cells than Capn1- and Capn2-knockdown cells. Together, these results suggest that the calpain small subunit plays an important role in the production of mechanical forces and in mediating mechanosensing during fibroblast migration. Furthermore, the Capn4 gene product might perform functions secondary to, or independent of, its role as a regulatory subunit for calpain 1 and calpain 2.
Figures




Similar articles
-
Reduced cell migration and disruption of the actin cytoskeleton in calpain-deficient embryonic fibroblasts.J Biol Chem. 2001 Dec 21;276(51):48382-8. doi: 10.1074/jbc.M108893200. Epub 2001 Oct 15. J Biol Chem. 2001. PMID: 11602605
-
Traction Force and Mechanosensing can be Functionally Distinguished Through the Use of Specific Domains of the Calpain Small Subunit.bioRxiv [Preprint]. 2023 Mar 9:2023.03.07.531592. doi: 10.1101/2023.03.07.531592. bioRxiv. 2023. PMID: 36945410 Free PMC article. Preprint.
-
Conditional disruption of ubiquitous calpains in the mouse.Genesis. 2006 Jun;44(6):297-303. doi: 10.1002/dvg.20216. Genesis. 2006. PMID: 16783822
-
The calpain system as a modulator of stress/damage response.Cell Cycle. 2007 Jan 15;6(2):136-8. doi: 10.4161/cc.6.2.3759. Epub 2007 Jan 27. Cell Cycle. 2007. PMID: 17264674 Review.
-
Regulating cell migration: calpains make the cut.J Cell Sci. 2005 Sep 1;118(Pt 17):3829-38. doi: 10.1242/jcs.02562. J Cell Sci. 2005. PMID: 16129881 Review.
Cited by
-
Galectin-3 secretion and tyrosine phosphorylation is dependent on the calpain small subunit, Calpain 4.Biochem Biophys Res Commun. 2011 Jun 24;410(1):91-6. doi: 10.1016/j.bbrc.2011.05.112. Epub 2011 May 27. Biochem Biophys Res Commun. 2011. PMID: 21640083 Free PMC article.
-
Obesity-associated dysregulation of calpastatin and MMP-15 in adipose-derived stromal cells results in their enhanced invasion.Stem Cells. 2012 Dec;30(12):2774-83. doi: 10.1002/stem.1229. Stem Cells. 2012. PMID: 22969001 Free PMC article.
-
Low-level stretching accelerates cell migration into a gap.Int Wound J. 2017 Aug;14(4):698-703. doi: 10.1111/iwj.12679. Epub 2016 Oct 17. Int Wound J. 2017. PMID: 27748039 Free PMC article.
-
Calpain activity is essential in skin wound healing and contributes to scar formation.PLoS One. 2012;7(5):e37084. doi: 10.1371/journal.pone.0037084. Epub 2012 May 16. PLoS One. 2012. PMID: 22615899 Free PMC article.
-
Serum Proteome Profiles in Stricturing Crohn's Disease: A Pilot Study.Inflamm Bowel Dis. 2015 Aug;21(8):1935-41. doi: 10.1097/MIB.0000000000000445. Inflamm Bowel Dis. 2015. PMID: 26199992 Free PMC article.
References
-
- Beckerle MC, Burridge K, DeMartino GN, Croal DE. Co-localization of calcium-dependent protease II and one of its substrates at sites of cell adhesion. Cell. 1987;51:569–577. - PubMed
-
- Beningo KA, Wang Y-L. Double hydrogel substrate as a model system for three-dimensional cell culture. Methods Mol. Biol. 2006;370:203–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

