Sorafenib for older patients with renal cell carcinoma: subset analysis from a randomized trial
- PMID: 18840822
- PMCID: PMC2567417
- DOI: 10.1093/jnci/djn319
Sorafenib for older patients with renal cell carcinoma: subset analysis from a randomized trial
Abstract
Background: The perception that older cancer patients may be at higher risk than younger patients of toxic effects from cancer therapy but may obtain less clinical benefit from it may be based on the underrepresentation of older patients in clinical trials and the known toxic effects of cytotoxic chemotherapy. It is not known how older patients respond to targeted therapy.
Methods: This retrospective subgroup analysis of data from the phase 3, randomized Treatment Approach in Renal Cancer Global Evaluation Trial examined the safety and efficacy of sorafenib in older (age >or=70 years, n = 115) and younger patients (age <70 years, n = 787) who received treatment for advanced renal cell carcinoma. Patient demographics and progression-free survival were recorded. Best tumor response, clinical benefit rate (defined as complete response plus partial response plus stable disease), time to self-reported health status deterioration, and toxic effects were assessed by descriptive statistics. Health-related quality of life was assessed with a Cox proportional hazards model. Kaplan-Meier analyses were used to summarize time-to-event data.
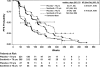
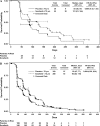
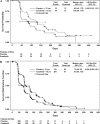
Results: Median progression-free survival was similar in sorafenib-treated younger patients (23.9 weeks; hazard ratio [HR] for progression compared with placebo = 0.55, 95% confidence interval [CI] = 0.47 to 0.66) and older patients (26.3 weeks; HR = 0.43, 95% CI = 0.26 to 0.69). Clinical benefit rates among younger and older sorafenib-treated patients were also similar (83.5% and 84.3%, respectively) and were superior to those of younger and older placebo-treated patients (53.8% and 62.2%, respectively). Adverse events were predictable and manageable regardless of age. Sorafenib treatment delayed the time to self-reported health status deterioration among both older patients (121 days with sorafenib vs 85 days with placebo; HR = 0.66, 95% CI = 0.43 to 1.03) and younger patients (90 days with sorafenib vs 52 days with placebo; HR = 0.69, 95% CI = 0.59 to 0.81) and improved quality of life over that time.
Conclusions: Among patients with advanced renal cell carcinoma receiving sorafenib treatment, outcomes of older (>or=70 years) and younger (<70 years) patients were similar.
Figures

References
-
- Scelo G, Brennan P. The epidemiology of bladder and kidney cancer. Clin Pract Urol. 2007;4((4)):205–217. - PubMed
-
- McLaughlin JK, Lipworth L, Tarone RE. Epidemiologic aspects of renal cell carcinoma. Semin Oncol. 2006;33((5)):527–533. - PubMed
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55((2)):74–108. - PubMed
-
- Repetto L, Balducci L. A case for geriatric oncology. Lancet Oncol. 2002;3((5)):289–297. - PubMed
-
- Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341((27)):2061–2067. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

